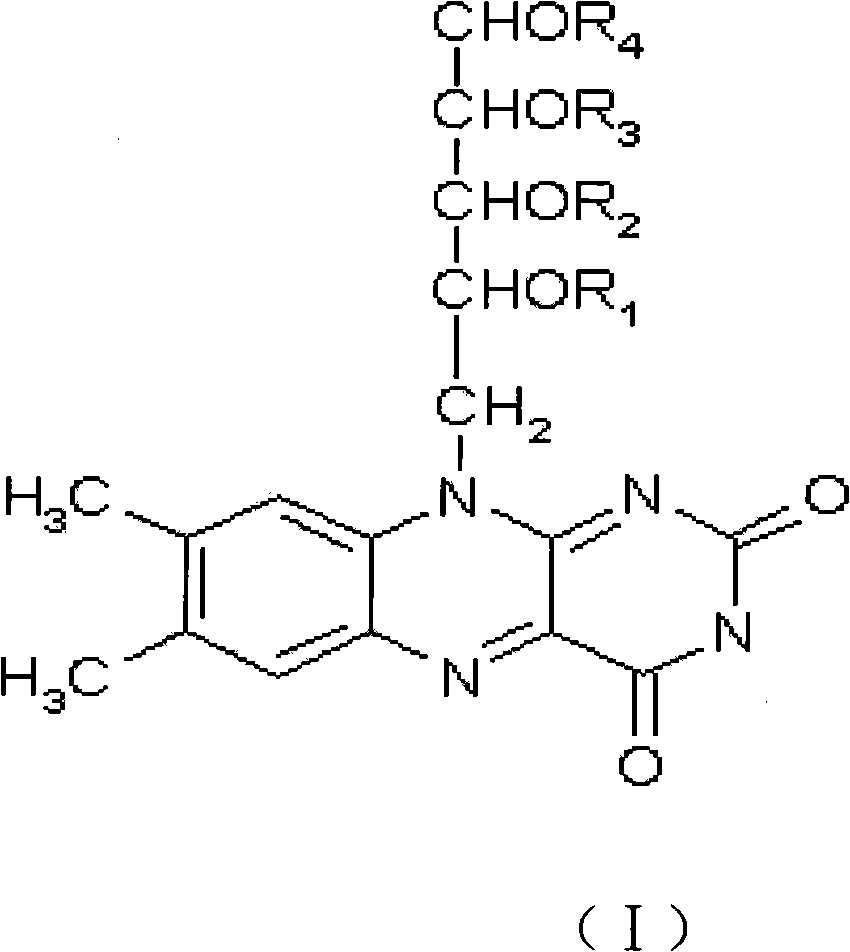
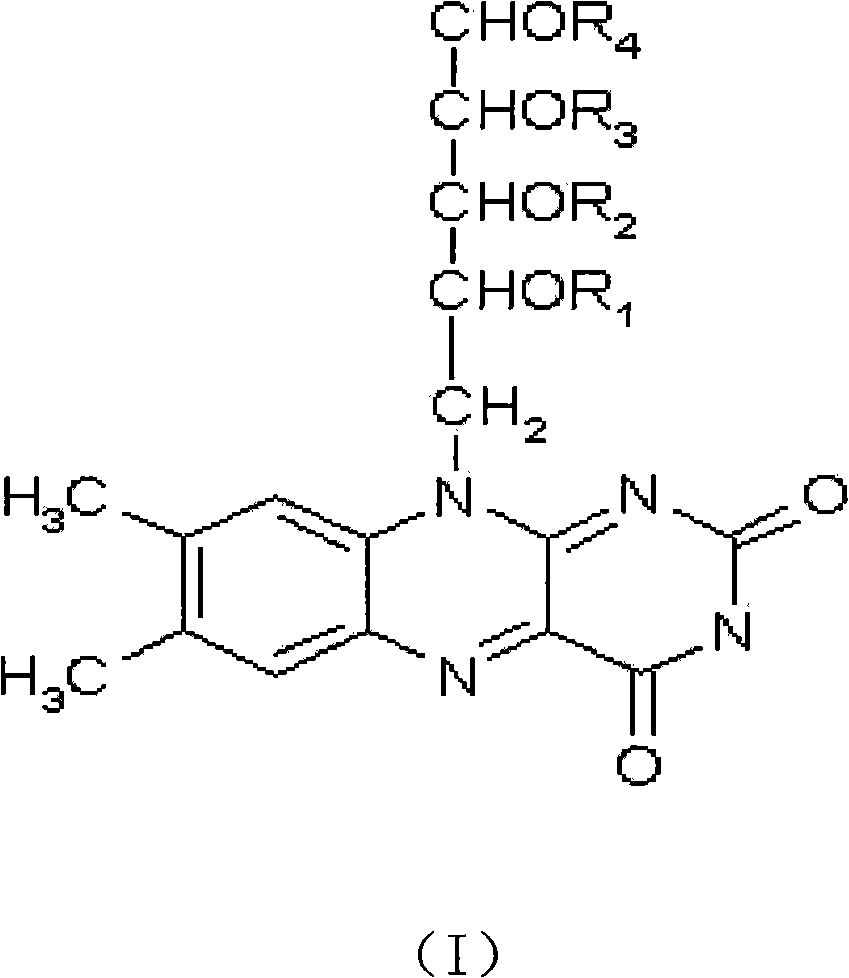
Application of lactoflavin ester derivative for preparing medicine for treating diabetes mellitus and complication thereof
A technology for riboflavin and diabetes, which is applied in the field of riboflavin ester derivatives for the preparation of drugs for treating diabetes and its complications, can solve the problem of limited storage capacity, and achieve a simple method and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
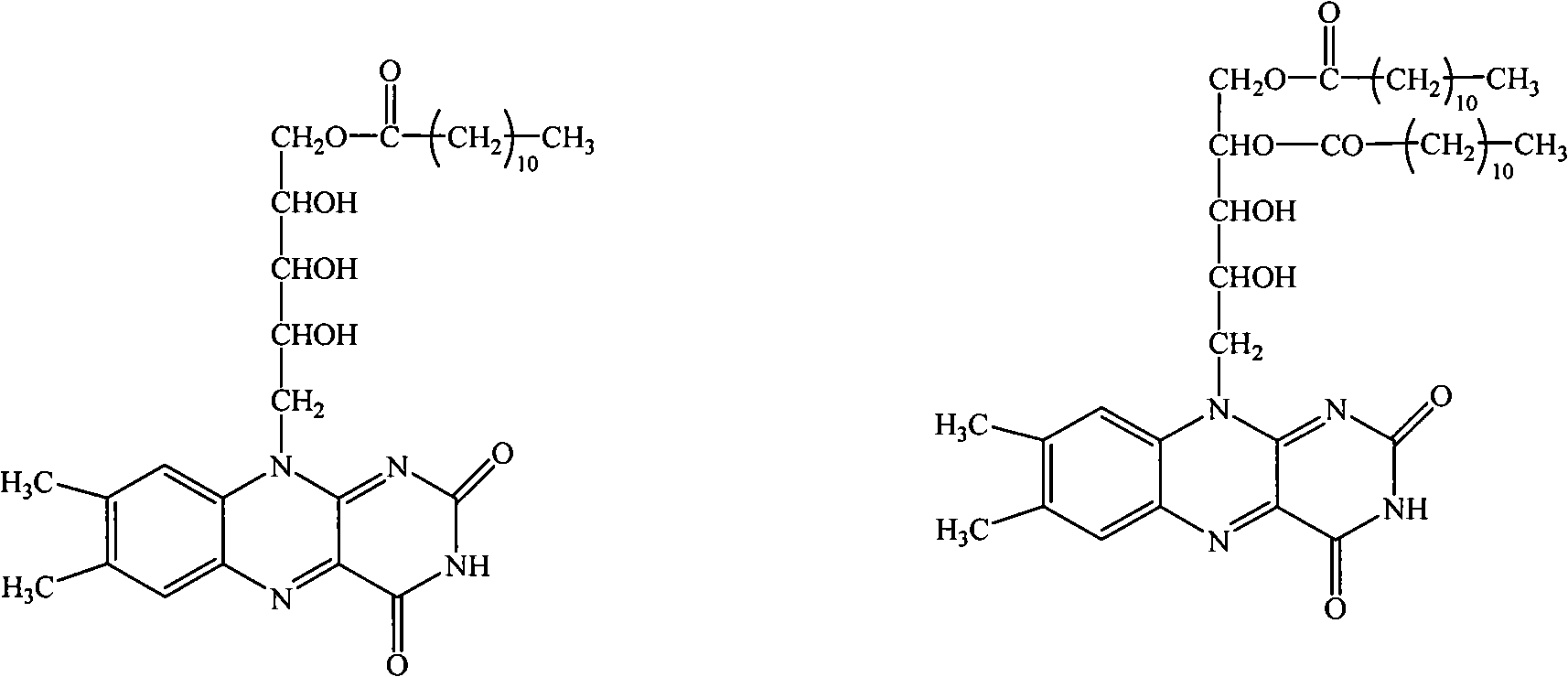
[0018] Embodiment 1: the preparation of riboflavin laurate
[0019] Weigh 28.8g of riboflavin (purchased from Hubei Guangji Pharmaceutical Co., Ltd., the same below) into a three-necked reaction flask, add 192ml of pyridine, heat to 100°C in an oil bath with stirring, add dropwise 18.6ml of lauroyl chloride and dissolve in 15- After the dropwise addition was completed within 20 minutes, continue to heat up to 118°C and reflux for 1.5 hours, then slowly add 18.6ml of lauroyl chloride dropwise until the reaction solution is transparent, cool and filter, and after the filtrate is concentrated under reduced pressure, add 240ml of methanol to reflux to precipitate crystals. Filtrate and dry at 75°C to obtain 18g of riboflavin laurate with a melting point of 23-245°C, dissolve in 180ml of DMF (dimethylformamide), and use 15×150cm LH 20 Layer column, after 2-3 times of separation and recrystallization, riboflavin-5'-lauric acid monoester, riboflavin-5', 4'-lauric acid diester and rib...
Embodiment 2
[0025] Embodiment 2: the synthesis of riboflavin isobutyrate
[0026] Suspend 5g of riboflavin in 50g of isobutyric anhydride (purchased from ACROS), stir and heat to 40°C, slowly add 6ml of concentrated sulfuric acid dropwise, and continue to stir and react at 50-60°C for 7 hours after the addition, the reaction solution It is transparent, cooled and filtered, the filtrate is poured into ice water and stirred to precipitate precipitates, filtered, washed with water for several times, then heated to dissolve with 40ml of methanol and filtered with a small amount of activated carbon, cooled to crystallize, and refined once with methanol to obtain riboflavin- Tetraisobutyrate. mp 125-128°C. Elemental analysis, calculated value (%) N8.53; measured value N8.32.
Embodiment 3
[0027] Embodiment 3: the preparation of riboflavin-2,6-dimethoxybenzoate
[0028] Weigh 5g of riboflavin and 11g of 2,6-dimethoxybenzoyl chloride (purchased from ACROS), put them into a conical flask, heat and stir at 80-90°C for 1 hour, add 10ml of methanol under cooling, pour into Yellow crystals were precipitated in 2L of water, filtered, washed with water, and dried, yielding about 11.4g of crude product. The crude product was dissolved in 80ml of pyridine and filtered, and the filtrate was poured into 2L of water to precipitate crystals, filtered and dried, and then recrystallized with methanol to obtain riboflavin-2,6-dimethoxybenzoate, mp162-166°C elemental analysis, Calculated (%): C61.58, H5.07, N5.42; found C61.41, H5.24, N5.29.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com