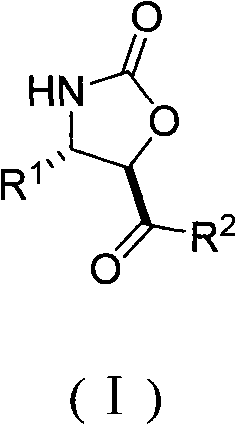
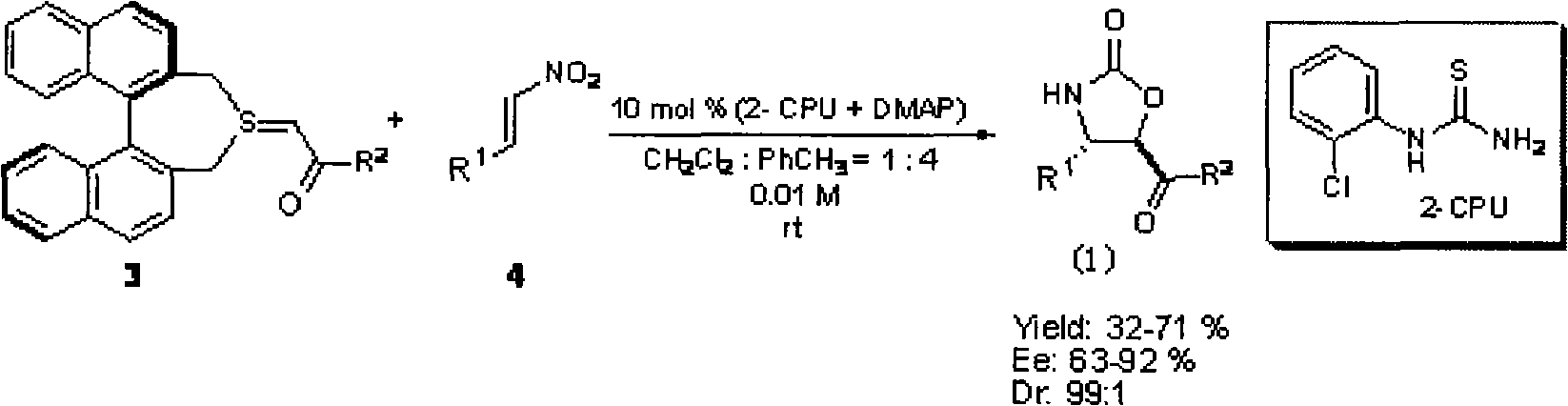Method for the effective synthesis of optically active oxazoline-2-ketone derivative
An optically active, oxazoline technology, used in the field of bioactivity of fungicides and herbicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] preparation of
[0019] At room temperature, in a 50mL round bottom flask, add 29.8mg trans-β-nitrostyrene (1 chemical equivalent (equiv), 0.2mmol), 3.74mg 2-CPU (10mol%, 0.02mmol), 2.44mg DMAP (10mol %, 0.02mmol) and 20mL solvent (V 甲苯 :V 二氯甲烷 = 4:1), stirring and dissolving, after half an hour, the reaction system turned pale yellow, and 107.6mg (1.25equiv, 0.25mmol) chiral sulfur ylide 3a was added to the system, and the reaction was continued until TLC (pure ethyl acetate was developed as Reagent) detection reaction is complete, column chromatography purification (V 石油醚 / V 乙酸乙酯 / V 二氯甲烷 =16:5:4), 38.0 mg of the target compound was obtained, with a yield of 71%.
[0020] Elemental analysis: measured value C% 72.13H% 5.14N% 5.58;
[0021] Calculated C% 71.90H% 4.90N% 5.24.
[0022] 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 7.94 (d, J = 8.0Hz, 2H), 7.61 (t, J = 7.2Hz, 1H), 7.47 (t, J = 6.4Hz, 2H), 7.44-7.34 (m, 5H), 6.30 (s, 1H), 5.46 (d, J=5.2Hz, 1H), 5.32 (d, J=5....
Embodiment 2
[0027] preparation of
[0028] At room temperature, add 35.8 mg trans-β-nitro-4-methoxystyrene (1 equiv), 0.2 mmol), 3.74 mg 2-CPU (10 mol%, 0.02 mmol), 2.44 mg DMAP into a 50 mL round bottom flask (10mol%, 0.02mmol) and 20mL solvent (V 甲苯 :V 二氯甲烷 = 4:1), stirring and dissolving, after half an hour, the reaction system turned pale yellow, and 107.6mg (1.25equiv, 0.25mmol) chiral sulfur ylide 3a was added to the system, and the reaction was continued until TLC (pure ethyl acetate was developed as Reagent) detection reaction is complete, column chromatography purification (V 石油醚 / V 乙酸乙酯 / V 二氯甲烷 =16:5:4), 32.1 mg of the target compound was obtained, with a yield of 54%.
[0029] Elemental analysis: measured value C% 68.83H% 5.30N% 4.59;
[0030] Calculated C% 68.68H% 5.09N% 4.71.
[0031] 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 7.94 (d, J = 7.6Hz, 2H), 7.62 (t, J = 7.6Hz, 2H), 7.47 (t, J = 7.6Hz, 2H), 7.33 (d, J = 8.0Hz, 2H), 6.93(d, J=8.4Hz, 2H), 5.88(s, 1H), 5.45(d, J=5.6...
Embodiment 3
[0036] preparation of
[0037]At room temperature, in a 50mL round bottom flask, add 36.7mg trans-β-nitro-4-chlorostyrene (1equiv, 0.2mmol), 3.74mg 2-CPU (10mol%, 0.02mmol), 2.44mg DMAP (10mol%) , 0.02mmol) and 20mL solvent (V 甲苯 :V 二氯甲烷 = 4:1), stirring and dissolving, after half an hour, the reaction system turned pale yellow, and 107.6mg (1.25equiv, 0.25mmol) chiral sulfur ylide 3a was added to the system, and the reaction was continued until TLC (pure ethyl acetate was developed as Reagent) detection reaction is complete, column chromatography purification (V 石油醚 / V 乙酸乙酯 / V 二氯甲烷 =16:5:4), 41.0 mg of the target compound was obtained, with a yield of 68%.
[0038] Elemental analysis: measured value C% 63.75 H% 3.65 N% 4.48;
[0039] Calculated for C% 63.69 H% 4.01 N% 4.64.
[0040] 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 7.94 (d, J = 7.6Hz, 2H), 7.63 (t, J = 7.2Hz, 1H), 7.48 (t, J = 7.6Hz, 2H), 7.38-7.32 (m, 4H), 6.62 (s, 1H), 5.39 (d, J=5.6Hz, 1H), 5.32 (d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com