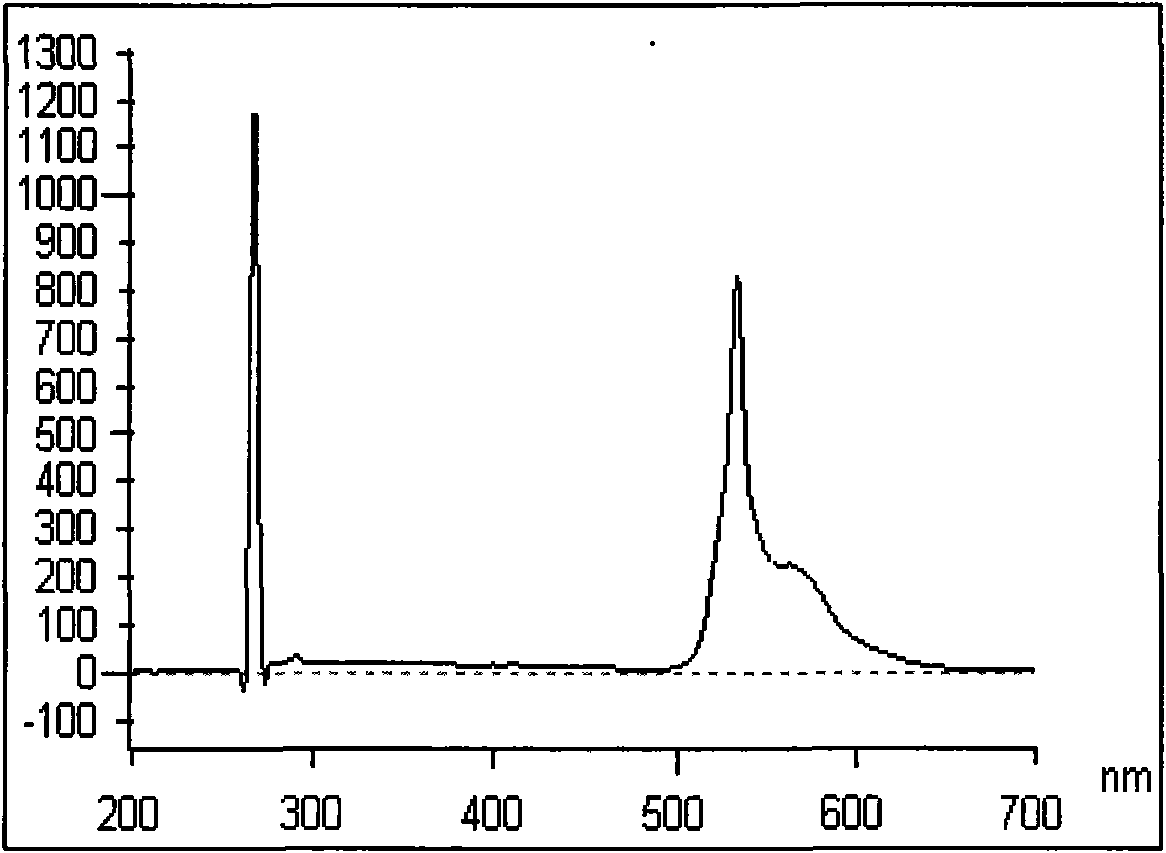
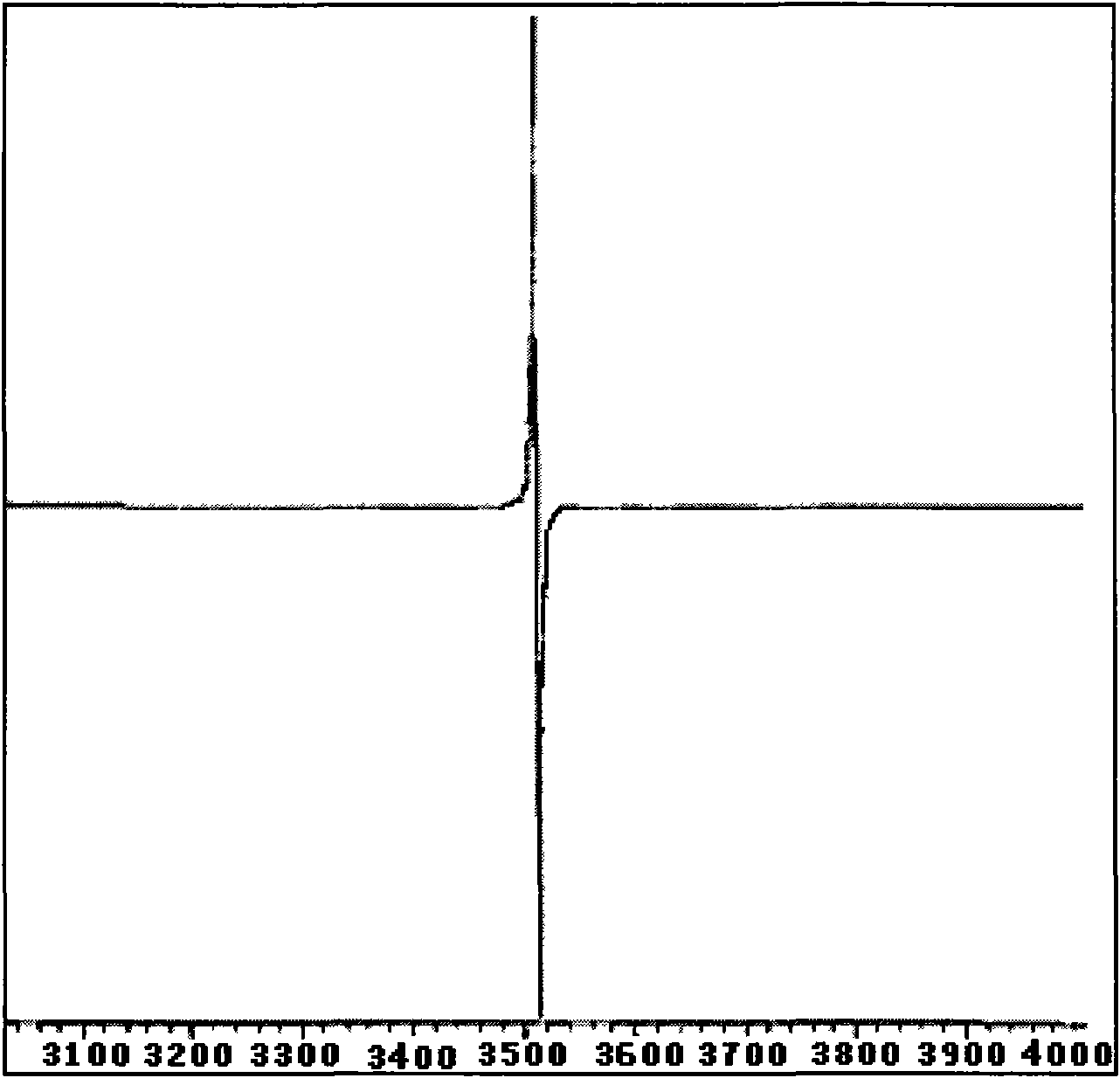
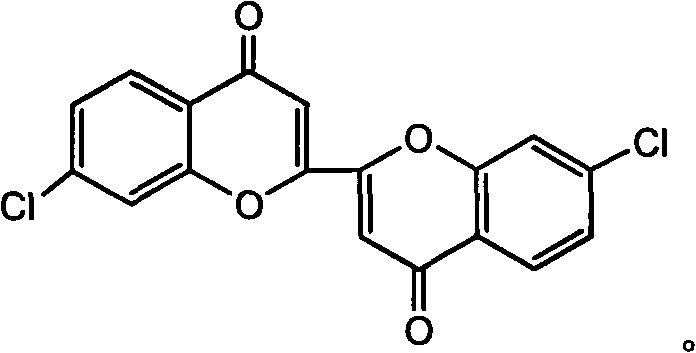
Solid magnetic and fluorescent organic compound of 4-chlorobiindenylisolactone and synthesis method thereof
A technology for chlorindene isolactone and organic compounds, which is applied in the field of organic compounds with dual functions of fluorescence and magnetic and their synthesis, and can solve problems such as weak signal and environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 250mL four-necked flask equipped with a thermometer, a reflux condenser and a mechanical stirrer, add 30.5g (0.167mol) of 4-chlorophthalic anhydride and succinic acid (1:1 in molar ratio), and heat up to 210°C. Start stirring, add potassium acetate (the molar ratio of potassium acetate to 4-chlorophthalic anhydride is 0.2:1), and react at 210°C for 2.5 hours. Then lower the temperature to 100°C, add hot water and introduce steam. Until the reaction product forms crushed particles, filter, wash the product with hot water, dry, and boil with ethanol to remove resinous impurities. Filter again, wash with ethanol until the eluate is colorless, and dry to obtain 18.3 g of reddish yellow powder, which is an intermediate for synthesizing 4-chloroindene isolactone.
[0029] In a 250mL four-neck flask equipped with a thermometer, a reflux condenser and a mechanical stirrer, suspend 18.0g of the intermediate with methanol, raise the temperature to 60°C, and drop the sodium...
Embodiment 2
[0031] In a 250mL four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 30.5g (0.167mol) of 4-chlorophthalic anhydride and succinic acid (molar ratio: 1:1.5), and heat up to 210°C . Start stirring, add potassium acetate (the molar ratio of 4-chlorophthalic anhydride to potassium acetate is 1:0.3), and react at 210°C for 2.5 hours. Then lower the temperature to 100°C, add hot water and introduce steam. Until the reaction product forms crushed particles, filter, wash the product with hot water, dry, and boil with ethanol to remove resinous impurities. Filter again, wash with ethanol until the eluate is colorless, and dry to obtain 15.6 g of reddish-yellow powder, which is an intermediate for the synthesis of 4-chloroindene isolactone.
[0032] In a 250mL four-neck flask equipped with a thermometer, a reflux condenser and mechanical stirring, suspend 15.0g of the intermediate with methanol, raise the temperature to 60°C, and drop the sodium ...
Embodiment 3
[0034]In a 250mL four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 30.5g (0.167mol) of 4-chlorophthalic anhydride and succinic acid (molar ratio: 1:2), and heat up to 210°C . Start stirring, add potassium acetate (the molar ratio of 4-chlorophthalic anhydride to potassium acetate is 1:0.3), and react at 210°C for 2.5 hours. Then lower the temperature to 100°C, add hot water and introduce steam. Until the reaction product forms crushed particles, filter, wash the product with hot water, dry, and boil with ethanol to remove resinous impurities. Filter again, wash with ethanol until the eluate is colorless, and dry to obtain 17.8 g of reddish-yellow powder, which is an intermediate for the synthesis of 4-chloroindene isolactone.
[0035] In a 250mL four-neck flask equipped with a thermometer, a reflux condenser and mechanical stirring, suspend 17.0g of the intermediate with methanol, raise the temperature to 60°C, and drop the sodium met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com