ING4 and IL-24 dual gene co-expression vector and application thereof
A CMV-IL-24, recombinant vector technology, applied in gene therapy, genetic engineering, plant genetic improvement and other directions, can solve problems such as inability to use, affecting the normal expression of dual genes, and no related reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
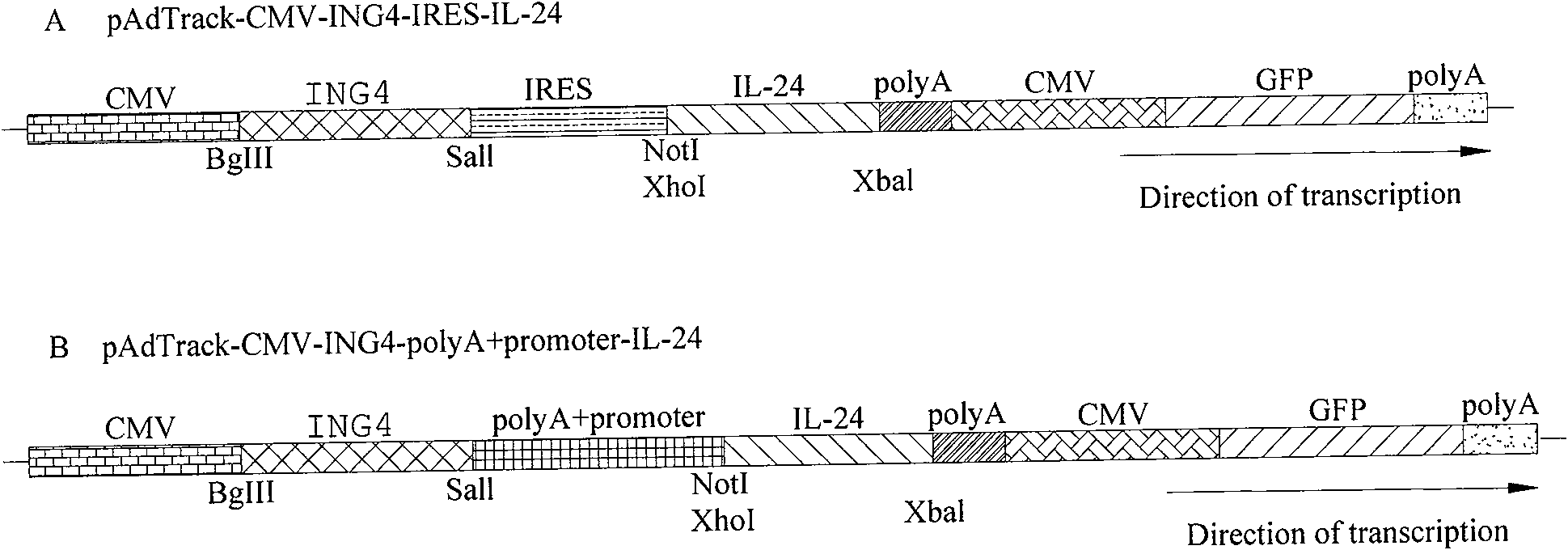
[0109] Embodiment one, the construction of each group of recombinant transfer vectors mediated by IRES
[0110] 1.1 Construction of pAdTrack-CMV-IRES Transformation Transfer Empty Vector
[0111] (1) Obtain the IRES target fragment by PCR: compare the conserved sequences of IRES, design primers P1 and P2 (as shown in Table 1), use the pGEZ-Term plasmid containing the IRES fragment as a template to amplify the IRES target fragment by PCR, and introduce SalI at both ends , NotI restriction site, the obtained IRES PCR product;
[0112] (2) Subcloning the IRES fragment into the pAdTrack-CMV transfer plasmid: use the IRES PCR product purified by the DNA cleaning kit and the transfer plasmid pAdTrack-CMV extracted by the daily mini-plasmid extraction kit, respectively, with SalI and NotI at 37°C with double enzymes After cutting for 5 hours, tap the gel to recover the target fragment, and use T4 DNA ligase to connect it overnight at 4°C, and then transform the ligation product into...
Embodiment 2
[0121] Example 2, polyA Δ296~298 +Construction of each group of recombinant transfer vectors mediated by CMV
[0122] 2.1pAdTrack-CMV-polyA Δ296~298 + CMV transformation transfer empty vector construction
[0123] (1) According to the reporter sequence of pORF-mbcl-2α plasmid, design primers P3, P4, P5, P6 (as shown in Table 1). Using the pORF-mbcl-2α plasmid as a template, P3, P4; P5, P6 as primers, PCR amplifies polyA and CMV target gene fragments (the size is consistent with the expected polyA and CMV theoretical value (333bp; 555bp) ); and with polyA and CMV mixed PCR product as template, P3, P6 as primer SOE-PCR amplification polyA+CMV fusion fragment (polyA+CMV fusion fragment of about 888bp size;
[0124] (2) Assembly of pUcm-T-polyA-CMV plasmid: Since the pAdTrack-CMV plasmid itself contains multiple PacI restriction sites, it is necessary to use PacI enzyme to cut the PacI restriction site in polyA-CMV, and then polymerize with T4DNA The base deletion method of en...
Embodiment 3
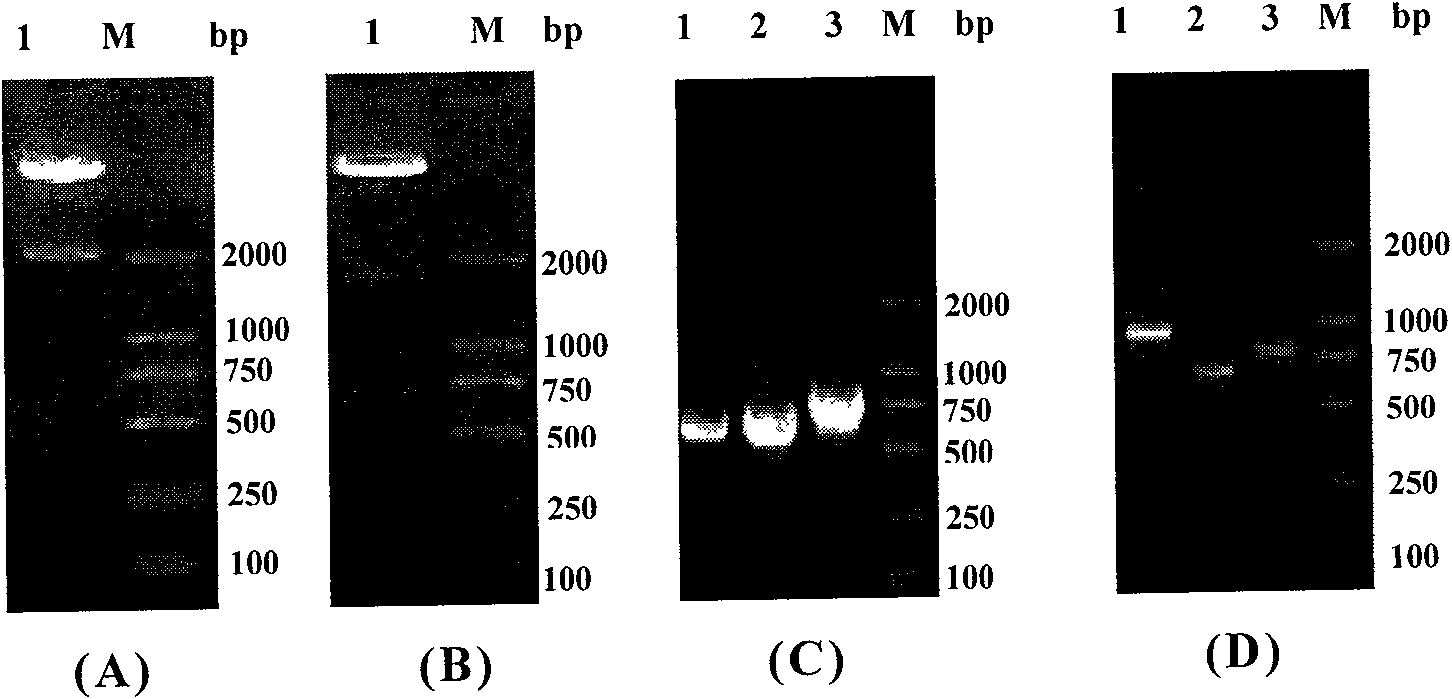
[0135] Example 3, construction and identification of homologous recombination adenoviral vector
[0136] The above pAdTrack-CMV-IRES, pAdTrack-CMV-IRES-IL-24, pAdTrack-CMV-ING4-IRES and pAdTrack-CMV-ING4-IRES-IL-24; pAdTrack-CMV-polyA Δ296~298 +CMV, pAdTrack-CMV-polyA Δ296~298 +CMV-IL-24, pAdTrack-CMV-ING4-polyA Δ296~298 +CMV and pAdTrack-CMV-ING4-polyA Δ296~298 +CMV-IL-24 recombinant transfer plasmid was linearized with PmeI at 37°C for 2 hours, then co-transformed BJ5183 competent bacteria with pAdEasy-1 adenovirus backbone plasmid by calcium chloride method, and LB containing Kana (50 μg / ml) Agar plate screening picks single clones to extract plasmids, and preliminarily screens pAdEasy-1-pAdTrack-CMV-IRES (abbreviated as pAd-IRES), pAdEasy-1-pAdTrack-CMV-IRES-IL-24 (abbreviated as pAdEasy-1-pAdTrack-CMV-IRES-IL-24) pAd-IRES-IL-24), pAdEasy-1-pAdTrack-CMV-ING4-IRES (referred to as pAd-ING4-IRES) and pAdEasy-1-pAdTrack-CMV-ING4-IRES-IL-24 (abbreviated as pAd -ING4-IRES-IL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com