Method for manufacturing alcohols
A manufacturing method and technology of alcohols, applied in the direction of organic chemical methods, chemical instruments and methods, and preparation of organic compounds, which can solve the problems of low yield and the use of a large amount of solvents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Hereinafter, although an Example and a comparative example are shown and this invention is demonstrated in detail, this invention is not limited to these.
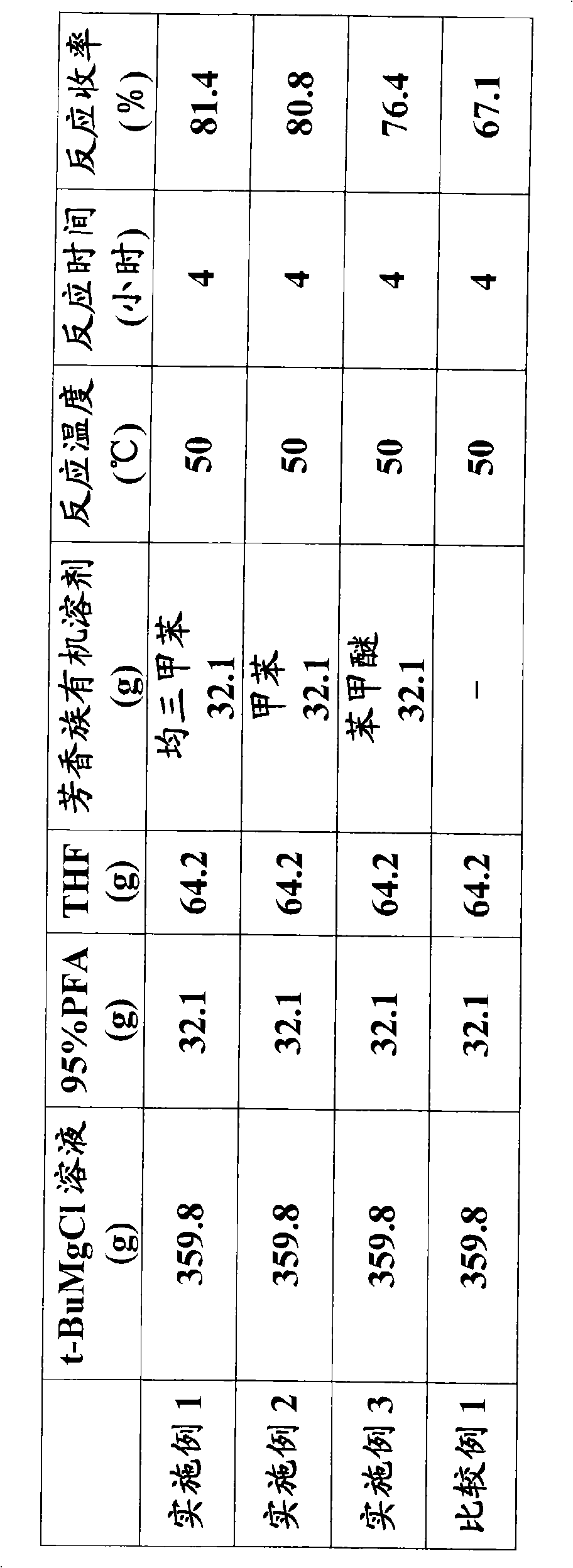
[0039] In a 500ml flask equipped with a stirrer, a condenser, a thermometer, and a dropping funnel, add 26.2g (1.08 moles) of magnesium metal and 233.6g of tetrahydrofuran. Add 100.0 g (1.08 moles) of tert-butyl chloride. After the dropwise addition, stir at an internal temperature of 50-55°C until the magnesium disappears to obtain tert-butylmagnesium chloride.
[0040] Next, in a 1000 ml flask equipped with a stirrer, a condenser, a thermometer, and a dropping funnel, 32.1 g (1.08 moles of pure components) of 95% paraformaldehyde (PFA), 32.1 g of mesitylene, and 64.2 g of tetrahydrofuran were added. , at an internal temperature of 45-50°C, add all the previously obtained tert-butylmagnesium chloride dropwise. After the dropwise addition, the mixture was stirred at an internal temperature of 45 to 50° C. for 4 ho...
Embodiment 2
[0046] Using the same apparatus as in Example 1, the reaction was carried out under the conditions shown in Table 1, substituting 32.1 g of toluene for mesitylene. The results are shown in Table 1.
Embodiment 3
[0048] Using the same apparatus as in Example 1, 32.1 g of anisole was used instead of mesitylene, and the reaction was carried out under the conditions shown in Table 1. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com