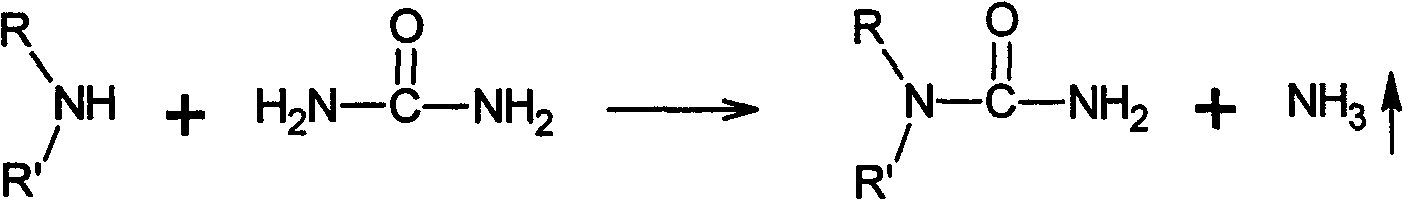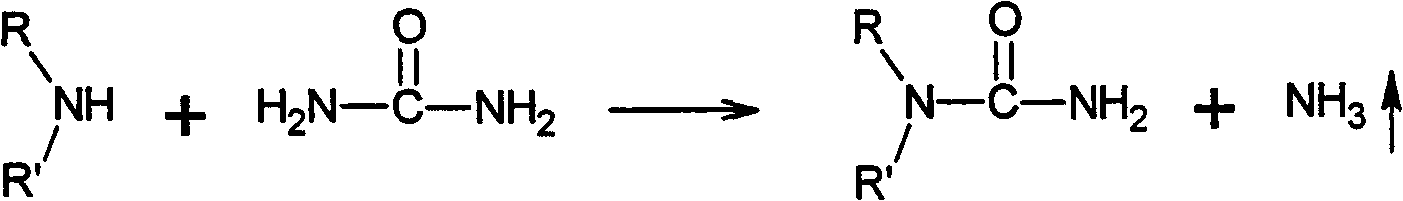Preparation method of meta-alkyl urea series compounds
A compound, alkyl urea technology, applied in the field of synthesis of organic compounds, can solve problems such as low product yield and purity, difficult steps to remove solvents, serious side reactions, etc., to reduce reaction costs, reduce risks, simplify the process The effect of the processing step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of N,N-diethylurea
[0045] Add 175g of urea, 250mL of diethylamine in a 1000mL four-necked bottle equipped with a stirrer, a thermometer and a reflux device, and the mol ratio of urea and amine is 1.2: 1.0; The volume ratio is 1:1. The preparation method of this water-based reaction medium is as follows: add 10g of zinc-zinc oxide complex, 8g of lead oxide-lead nitrate complex, and 2.5g of diethylammonium chloride into 250mL of pure water successively, stir to form a suspension, and The pH value is between 6 and 7.
[0046] The reaction system is stirred and heated to 60°C, and the overflowing ammonia gas is absorbed by the acid through the tail gas absorption device. After 60h, the reaction ended.
[0047] After the reaction was completed, the reactor was cooled to room temperature, and the crude product was fully dried and filtered to separate the insoluble matter. The resulting filtrate was recrystallized to obtain the pure product as white needle-like...
Embodiment 2
[0049] N,N-Dipropylurea Synthesis
[0050] Add 165g of urea, 250mL of dipropylamine in a 1000mL four-necked flask equipped with a stirrer, a thermometer and a reflux device, the molar ratio of urea and amine is 1.5:1.0; 125mL of water-based reaction medium, the volume of amine and water-based reaction medium The ratio is 2:1. The preparation method of the water-based reaction medium is as follows: add 10g of zinc-zinc oxide complex, 10g of ferric oxide-ferric chloride complex, and 1.5g of dipropylammonium chloride into 125mL of pure water in sequence, and stir to form a suspension solution with a pH between 6 and 7.
[0051] The reaction system is stirred and heated to 90°C, and the overflowing ammonia gas is absorbed by the acid through the tail gas absorption device. After 35h, the reaction ended.
[0052] After the reaction was completed, the reactor was cooled to room temperature, and the crude product was fully dried and filtered to separate the insoluble matter. The ...
Embodiment 3
[0054] Synthesis of N,N-Dibutylurea
[0055] Add 135g of urea, 250mL of dibutylamine in a 1000mL four-necked flask equipped with a stirrer, a thermometer and a reflux device, and the molar ratio of urea and amine is 1.5:1.0; 100mL of water-based reaction medium, amine and water-based reaction medium The volume ratio is 2.5:1. The preparation method of the water-based reaction medium is as follows: add 17g of zinc-zinc oxide-zinc acetate complex, 15g of stannous chloride, and 1g of dibutylammonium chloride into 100mL of pure water in turn, stir to form a suspension, and its pH The value is between 6 and 7.
[0056] The reaction system is stirred and heated to 120°C, and the overflowing ammonia gas is absorbed by the acid through the tail gas absorption device. After 24h, the reaction ended.
[0057] After the reaction was completed, the reactor was cooled to room temperature, and the crude product was fully dried and filtered to separate the insoluble matter. The resulting ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com