Method for preparing anilines diazosalt solid powder
A technology for aniline compounds and diazonium salts, applied in the field of intermediate preparation of synthetic dyes, can solve problems such as product quality decline, decomposition and explosion, production process danger, etc., achieve good stability, avoid explosion danger, and solve product quality problems falling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
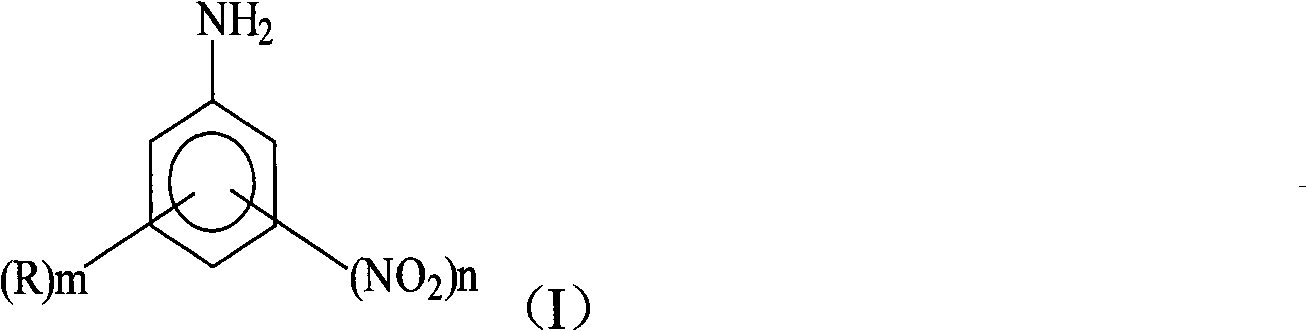
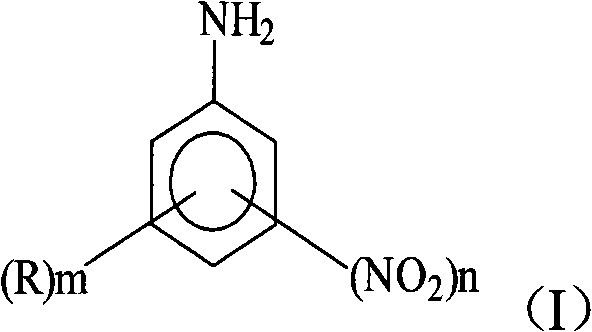
[0032] (1) Diazotization: Take 135.3g of anhydrous sulfuric acid and slowly add dried NaNO at a temperature of 25°C 2 11.2g, reacted at 80°C for 2 hours. Cool down to below 40°C, then add 28g of 2,4-dinitroaniline with a content of 98% at 40°C, react for 6 hours, cool down to 10°C, dilute into ice-water mixture, control the temperature to ≤0°C, and obtain 2, 4-Dinitroaniline diazonium sulfuric acid aqueous solution.
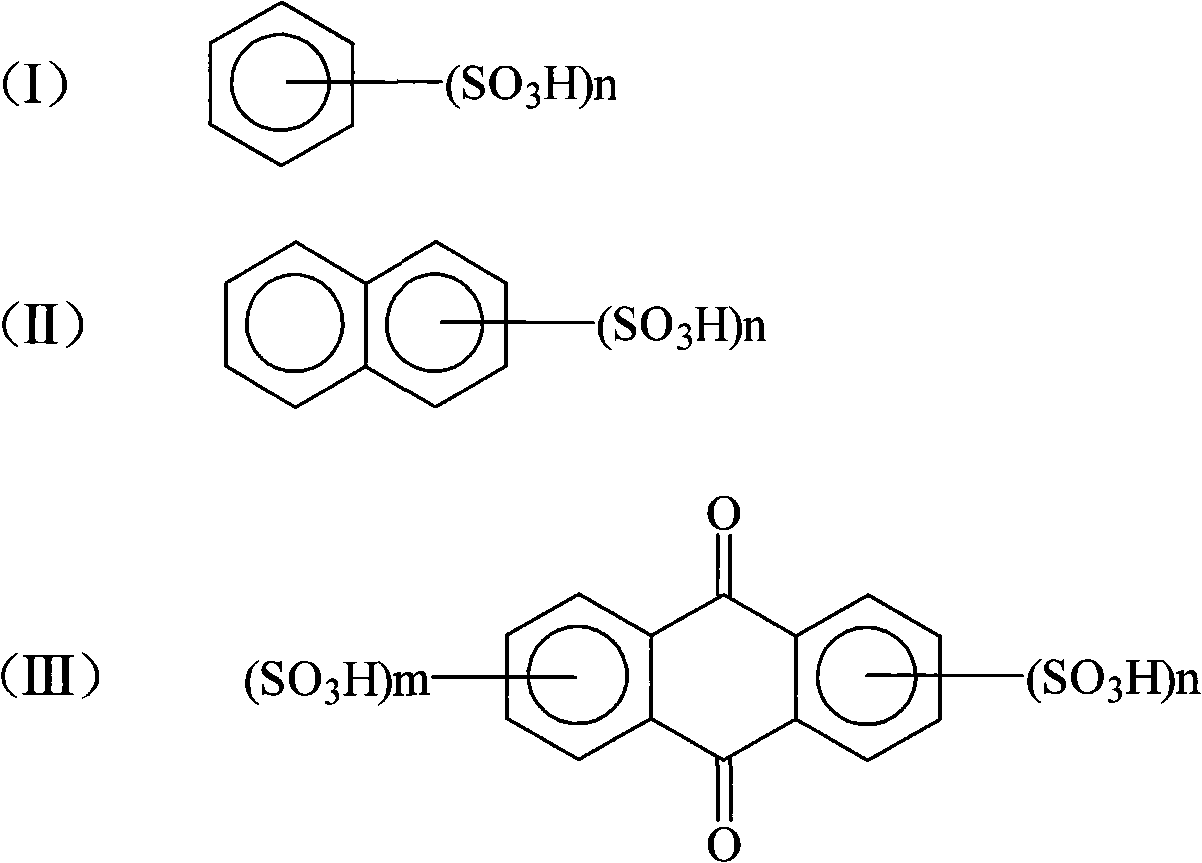
[0033] (2) Crystallization: In 2,4-dinitroaniline diazonium salt sulfuric acid aqueous solution, add 50g of benzenesulfonic acid, stir and precipitate for more than 2 hours, filter to get 2,4-dinitroaniline diazonium salt filter cake , to dry.
[0034] (3) Drying: Dry the filter cake of 2,4-dinitroaniline diazonium salt at about 80°C to obtain powdery 2,4-dinitroaniline diazonium salt, that is, the weight of 2,4-dinitroaniline Nitrogen salt solid powder.
Embodiment 2
[0036] (1) Diazotization: take 93.7g of 98% sulfuric acid, and mix it with 41.6g containing SO under cooling 3 20% oleum was mixed to make 135.3g of anhydrous sulfuric acid. In the anhydrous sulfuric acid, slowly add dried NaNO at a temperature ≤30°C 2 11.2g, reacted at 60°C for 3 hours. Cool down to below 40°C, then add 32g of 6-bromo-2,4-dinitroaniline with a content of 98% at 45°C, react for 8 hours, cool down to below 15°C, dilute into ice-water mixture, control temperature ≤ 0 °C to obtain 6-bromo-2,4-dinitroaniline diazonium sulfuric acid aqueous solution.
[0037] (2) crystallization: in 6-bromo-2,4-dinitroaniline diazonium sulfuric acid aqueous solution, add 35g of 1,5-naphthalene disulfonic acid and 15g sodium dodecylsulfonate, stir and separate out 2 After more than 1 hour, filter cake of diazonium salt of 6-bromo-2,4-dinitroaniline to be dried.
[0038] (3) Drying: Dry the filter cake of 6-bromo-2,4-dinitroaniline diazonium salt at 80°C to obtain powdery 6-bromo...
Embodiment 3
[0040] (1) Diazotization: take 93.7g of 98% sulfuric acid, and mix it with 41.6g containing SO under cooling 3 20% oleum was mixed to make 135.3g of anhydrous sulfuric acid. In the anhydrous sulfuric acid, slowly add dried NaNO at a temperature ≤30°C 2 11.2g, reacted at 70°C for 2 hours. Cool down to below 40°C, then add 20g of aniline with a content of 98% at 35°C, react for 8 hours, cool down to below 15°C, dilute into ice-water mixture, and control the temperature to ≤0°C to obtain aniline diazonium sulfuric acid aqueous solution.
[0041] (2) Crystallization: Add 40 g of anthraquinone-2,5-disulfonic acid to the aqueous solution of aniline diazonium salt sulfuric acid, stir and precipitate for more than 2 hours. Filter cake of 2,4-dinitroaniline diazonium salt and wait for drying.
[0042] (3) Drying: the aniline diazonium salt filter cake is dried below 80° C. to obtain powdery aniline diazonium salt, that is, aniline diazonium salt solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com