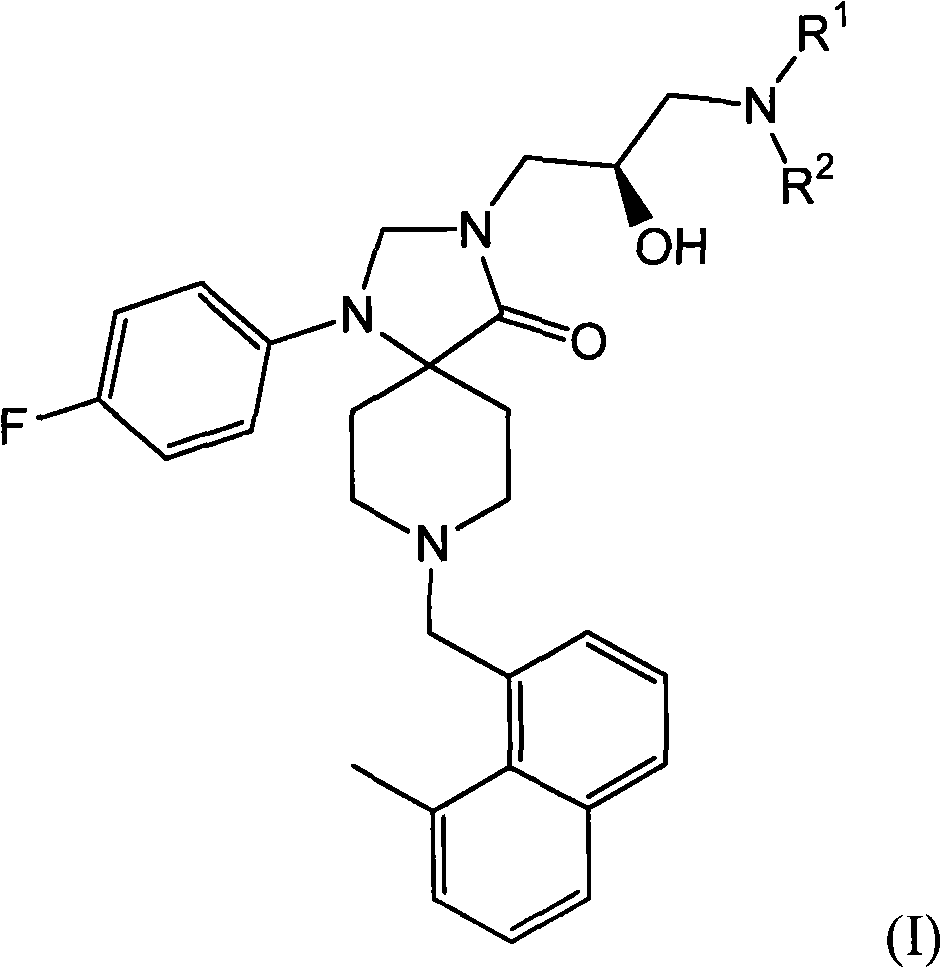
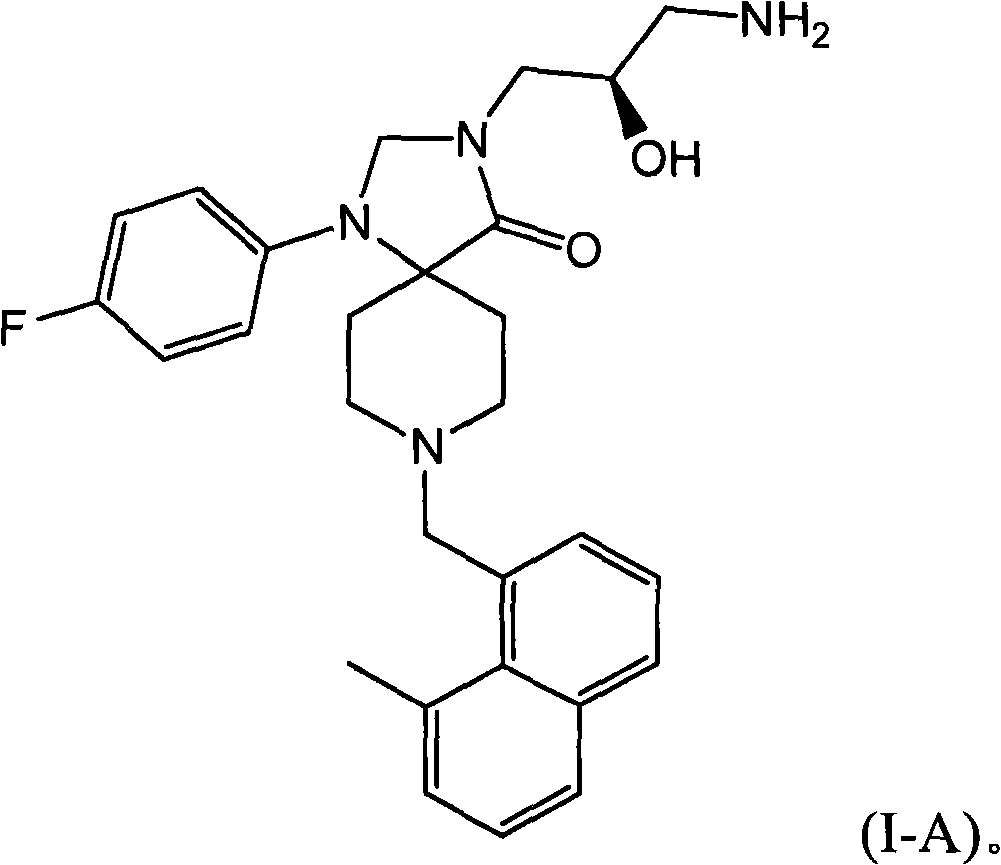
Methods for the treatment of alcohol abuse, addiction and dependency
A technology for alcohol abuse and alcohol dependence, which can be applied in pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., and can solve a large number of liver metabolism problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1-(4-fluoro-phenyl)-8-(8-methyl-naphthalen-1-ylmethyl)-1,3,8-triaza-spiro[4.5]decane-4-one
[0066]
[0067] Step A: (8-Hydroxymethyl-naphthalen-1-yl)-methanol
[0068] 1,8-Naphthalic anhydride (200 g, 1.0 mol) in toluene (2.5 L) was charged at room temperature in a 12-L vessel equipped with a thermocouple, overhead stirrer, 2-L additional funnel, and condenser under nitrogen. -L4 neck flask. DIBAL-H (1.5 M in toluene, 2.664 L, 4 mol) was added via an additional funnel over 1.5 h while the reaction mixture was stirred. The solution was then heated to 95°C overnight, cooled to 15°C, and washed with ethyl acetate (2.2 L) and H 2 O (2 L) was diluted slowly, followed by the addition of conc. HCl (320 mL). The resulting suspension was stirred at room temperature for 30 min, filtered and air-dried on filter paper for 2 h. The resulting material was stirred in 95% ethanol (1.2 L) at 70°C for 2 h, filtered to obtain a wet solid, which was air-dried on filter paper ov...
Embodiment 2
[0087] 1-(4-fluoro-phenyl)-8-(8-methyl-naphthalen-1-ylmethyl)-3-(S)-oxiranylmethyl-1,3,8-triazine Hetero-spiro[4.5]decane-4-one
[0088]
[0089] 1-(4-fluoro-phenyl)-8-(8-methyl-naphthalen-1-ylmethyl)-1,3,8-triaza-spiro[4.5]-decane-4-one (2.0 g, 4.95 mmol) was dissolved in N,N-dimethylformamide (25.0 mL). Sodium hydride (60% in mineral oil, 238 mg, 5.94 mmol) was then added to the reaction mixture at 0°C under nitrogen atmosphere, and the reaction mixture was stirred at 0°C for one hour. Then (2R)-(-)-3-nitrobenzenesulfonic acid glycidyl ester (1.54 g, 5.94 mmol) was added to the reaction mixture at 0°C. The reaction mixture was stirred at 0°C for one hour, then at room temperature under nitrogen for 18 hours, and partitioned with water and ethyl acetate. The organic layer was washed with brine, washed with Na 2 SO 4 Dry, filter and evaporate the solvent in vacuo to give a crude oil. The crude oil was purified by flash chromatography (2.5% methanol / dichloromethane)...
Embodiment 3
[0093] 3-(3-Amino-2-(R)-hydroxy-propyl)-1-(4-fluoro-phenyl)-8-(8-methyl-naphthalene-1-ylmethyl base)-1,3,8-triaza-spiro[4.5]decane-4-one
[0094]
[0095] 1-(4-fluoro-phenyl)-8-(8-methyl-naphthalen-1-ylmethyl)-3-(S)-oxiranylmethyl-1,3,8-triazine Hetero-spiro[4.5]decan-4-one (0.06 g, 0.13 mmol) was dissolved in ethanol (2 mL) and methanol (0.4 mL). Concentrated ammonium hydroxide (1 mL) was then added to the solution and the reaction mixture was stirred in a pressure bottle at 40°C for two hours. The solvent was then evaporated in vacuo to give a crude oil. The crude oil was purified by flash chromatography (5.0% ammonia 2.0 M in methanol / dichloromethane) to afford the title compound as a foam.
[0096] 1 H NMR (400MHz, CDCl 3 )δ7.77-7.75 (1H, m), 7.71-7.68 (1H, m), 7.37-7.30 (4H, m), 6.97-6.91 (2H, m), 6.87-6.83 (2H, m), 4.74 ( 2H, s), 4.0 (2H, s), 3.79-3.74 (1H, m), 3.57-3.52 (1H, m), 3.41-3.36 (1H, m), 3.11 (3H, s), 2.91-2.74 ( 4H, m), 2.66-2.61 (1H, m), 2.30-2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com