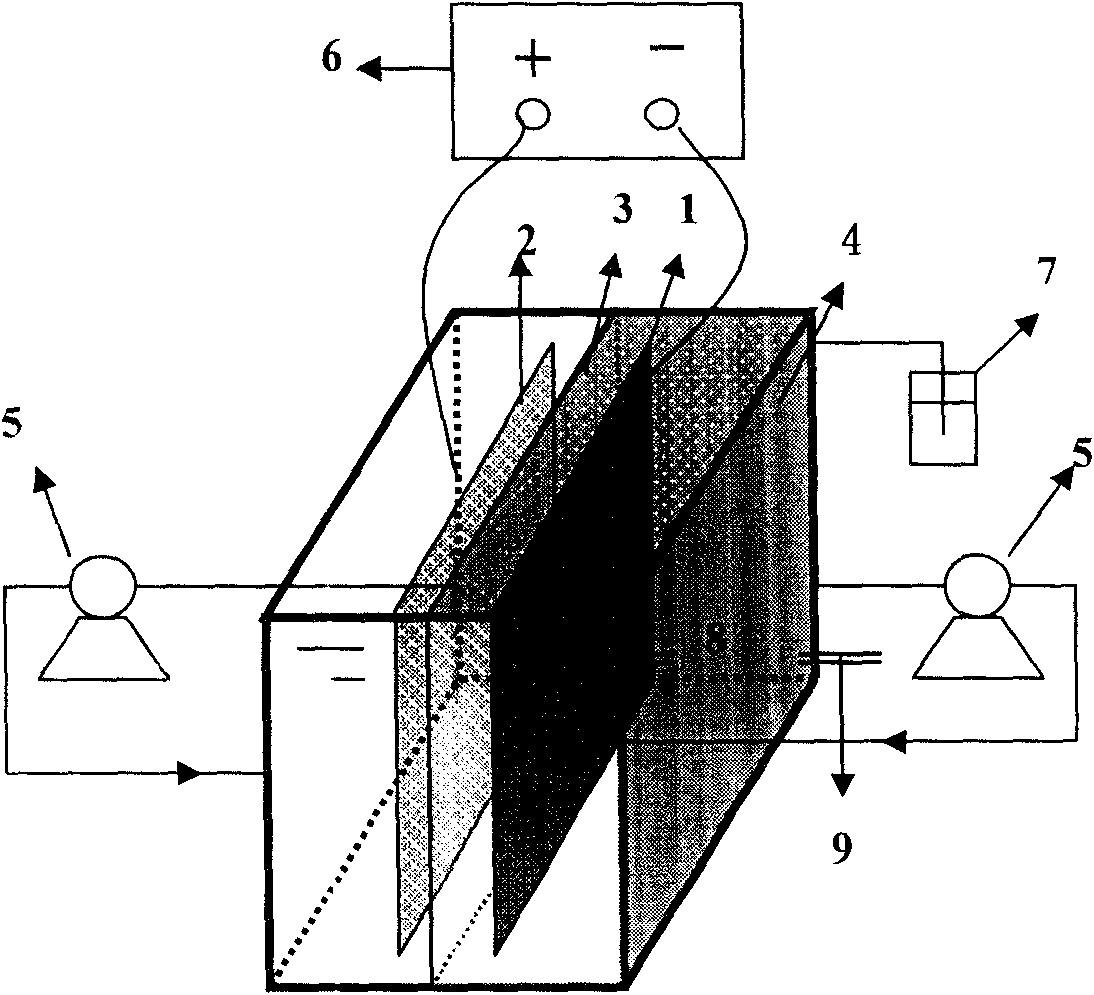
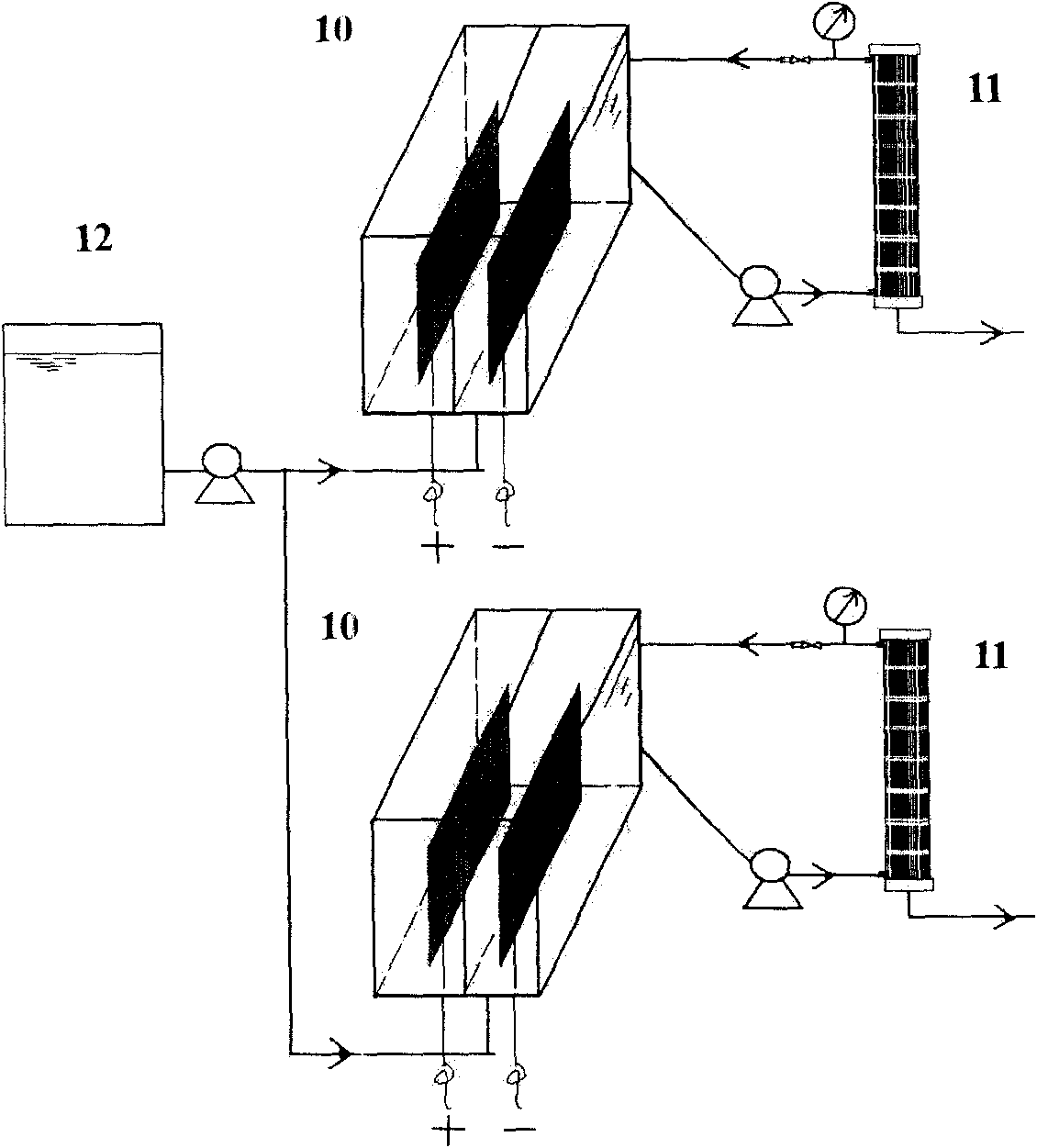
Method and reactor for removing nitrate through catalytic electrochemical biological hydrogen autotrophic denitrification
An autotrophic denitrification and nitrate technology, applied in chemical instruments and methods, electrochemical water/sewage treatment, biological water/sewage treatment, etc., can solve problems such as low current efficiency, and achieve high current efficiency and biological treatment units. Simplified, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The effective volume of the cathode chamber of the reactor is 400mL. Surface modification of copper electrode with self-made catalytic electrode Pd (galvanostatic electrodeposition, PdCl 2 Concentration: 0.005mol / L; NH 4 Cl concentration: 0.1mol / L; deposition time: 15min; pH: 0.3) as the cathode, keep the hydrogen autotrophic denitrification sludge concentration of 700mg / L in the cathode chamber, apply a current of 50mA, when NO 3 - The initial concentration is 100mg / L, after 3.5h of reaction, the reactor NO 3 - -N removal rate > 95%, and no NO 2 - and NH 4 + accumulation.
Embodiment 2
[0022] The effective volume of the cathode chamber of the reactor is 400mL. Surface modification of copper electrode with self-made catalytic electrode Pd (galvanostatic electrodeposition, PdCl 2 Concentration: 0.005mol / L; NH 4 Cl concentration: 0.1mol / L; deposition time: 15min; pH: 0.3) as the cathode, keep the concentration of hydrogen autotrophic denitrification sludge in the cathode chamber at 1000mg / L, apply a current of 100mA, when NO 3 - The initial concentration is 100mg / L, after 2.5h of reaction, the reactor NO 3 - -N removal rate > 95%, and no NO 2 - and NH 4 + accumulation.
Embodiment 3
[0024] The effective volume of the cathode chamber of the reactor is 400mL. Surface modification of copper electrode with self-made catalytic electrode Pd (galvanostatic electrodeposition, PdCl 2 Concentration: 0.005mol / L; NH 4 Cl concentration: 0.1mol / L; deposition time: 15min; pH: 0.3) as the cathode, keep the concentration of hydrogen autotrophic denitrification sludge in the cathode chamber at 1000mg / L, apply a current of 50mA, when NO 3 - The initial concentration is 70mg / L, after 2.0h of reaction, the reactor NO 3 - -N removal rate > 95%, and no NO 2 - and NH 4 + accumulation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com