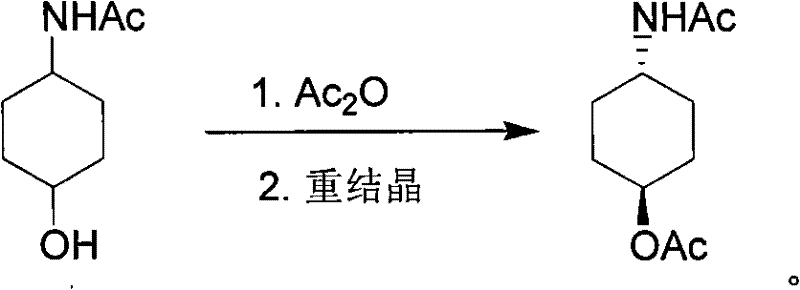
Method for preparing trans-4-acetamido-cyclohexanol acetic ester
A technology of acetamido, alcohol acetate, applied in the preparation of carboxylic acid amide optical isomers, organic chemistry, etc., can solve problems such as adding reaction steps, and achieve the effects of simple raw materials, easy operation and high crystallization yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The molar ratio of the feed material is 4-acetylaminocyclohexanol:acetic anhydride=1.0:1.0. Wherein the 4-acetylaminocyclohexanol cis-trans ratio is 30:70.
[0029] Add acetic anhydride (102g, 1.0mol) in the reaction flask, add 4-acetylaminocyclohexanol (157g, 1.0mol) under stirring, the reaction system is exothermic, after the natural temperature rise of the reaction mixture system slows down, heat to reflux reaction 2 hours later. Cool to 85°C, and recover the generated acetic acid by distillation under reduced pressure. The remaining solid was added to 500mL ethyl acetate, heated to reflux until the solid was dissolved, cooled to about 10°C for recrystallization, and 79.8 g of filtered white solid trans-4-acetylaminocyclohexanol acetate, with a molar yield of 40.1% ( Based on the amount of the cis-trans mixture in the raw material, the molar yield=the amount of trans-4-acetamidocyclohexanol acetate / 4-acetamidocyclohexanol in the examples) -The yield of isomer cont...
Embodiment 2
[0031] The molar ratio of the feed material is 4-acetylaminocyclohexanol:acetic anhydride=1.0:1.1. Wherein the 4-acetylaminocyclohexanol cis-trans ratio is 30:70.
[0032] Add acetic anhydride (112g, 1.10mol) in the reaction flask, add 4-acetylaminocyclohexanol (157g, 1.0mol) under stirring, the reaction system exotherms, after the natural temperature rise of the reaction mixture system slows down, heat to reflux reaction 2 Hour. Cool to 85°C, and recover the generated acetic acid and excess acetic anhydride by distillation under reduced pressure. Add 500mL ethyl acetate to the remaining solid, heat and reflux until the solid dissolves, cool to about 5°C to crystallize, and filter the white solid trans-4-acetylaminocyclohexanol acetate 83.9g, the molar yield is 42.2% (based on raw material The yield in terms of trans-isomer content is 60.3%), the melting point is 158-161°C, and the liquid phase purity is 99.3%.
Embodiment 3
[0034] The molar ratio of the feed material is 4-acetylaminocyclohexanol:acetic anhydride=1.0:1.2. Wherein the 4-acetylaminocyclohexanol cis-trans ratio is 30:70.
[0035] Add acetic anhydride (122g, 1.2mol) in the reaction flask, add 4-acetylaminocyclohexanol (157g, 1.0mol) under stirring, the reaction system is exothermic, after the natural temperature rise of the reaction mixed system slows down, heat to reflux reaction 2 Hour. Cool to 85°C, and recover the generated acetic acid and excess acetic anhydride by distillation under reduced pressure. Add 500mL ethyl acetate to the remaining solid, heat to reflux until the solid dissolves, cool to 15°C to crystallize, and filter the white solid trans-4-acetamidocyclohexanol acetate 95.5g, molar yield 48% (from the raw material Trans-isomer content yield 68.6%), melting point 158-161 ° C, liquid phase purity 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com