Anti-hepatofibrosis traditional Chinese medicament composition, preparation method thereof and medicament preparation
A technology of anti-hepatic fibrosis and composition, which is applied in the field of Chinese patent medicine and its preparation, can solve the problems of high price, single effect, and insignificant curative effect, and achieve the effect of improving liver protection and preventing fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 3
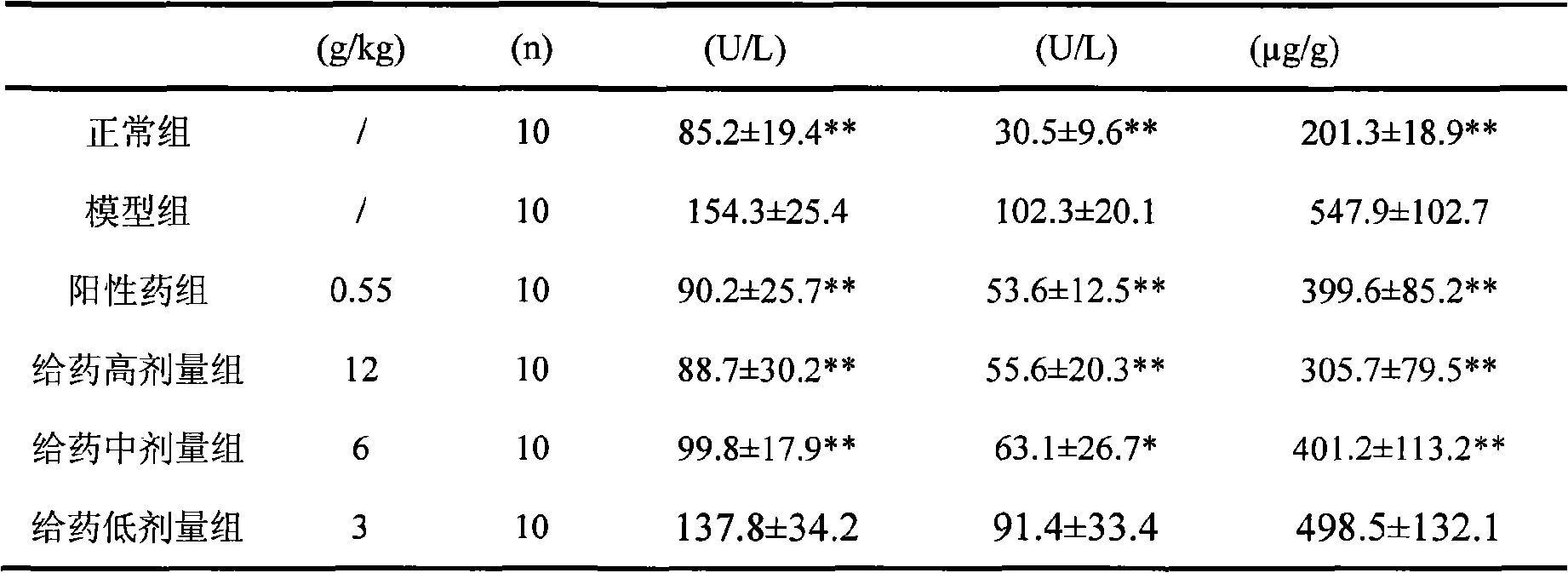
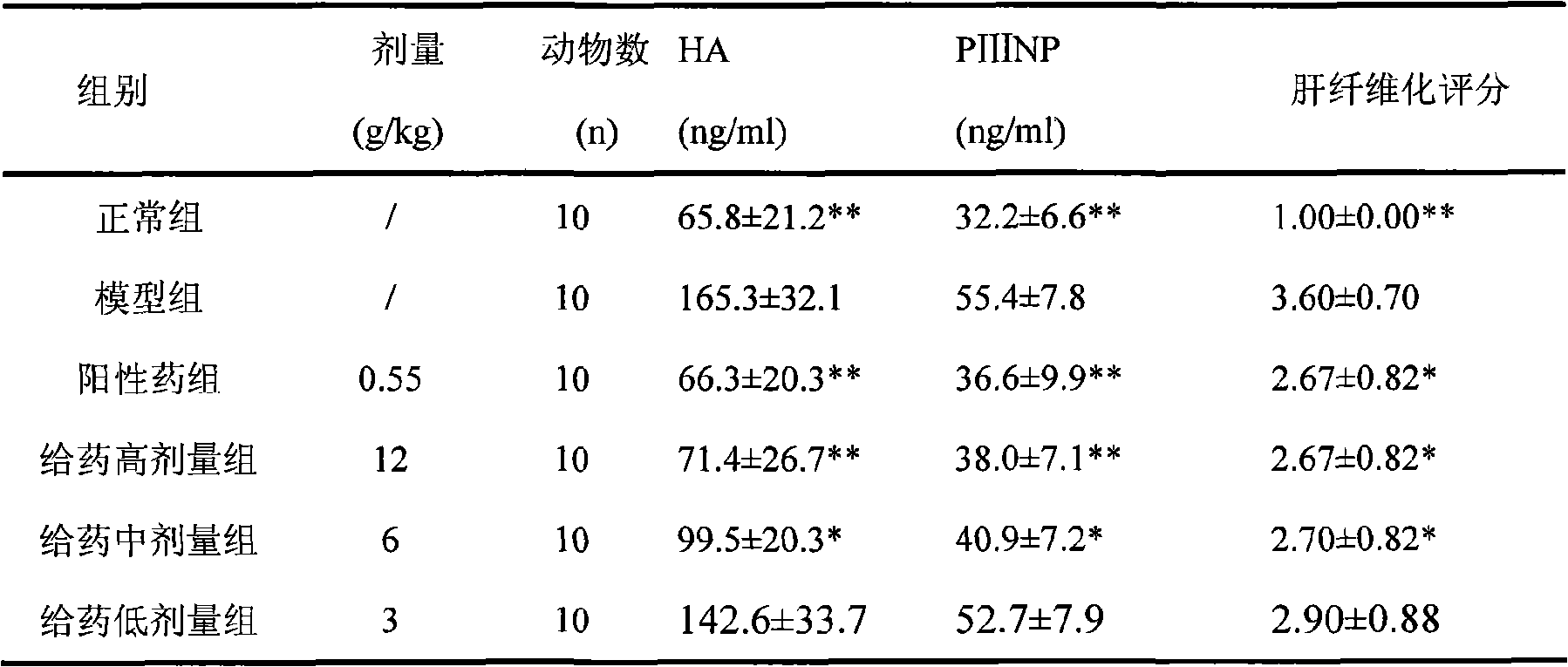
[0091] Experimental example 3 Chinese medicine composition of the present invention is to the hepatoprotective action test of mouse
[0092] Experimental method: 60 clean-grade KM mice, weighing 18-20g, half male and half male, were randomly divided into 6 groups, namely normal control group, model group, positive drug group and high, medium and low dose administration groups. Both the normal and model groups were intragastrically given equal volumes of distilled water, the high, middle and low dose groups were given the drug of the present invention 24g crude drug / kg, 12g crude drug / kg, and 6g crude drug / kg respectively, and the positive drug group was given compound soft-shelled turtle A soft liver tablet 1.1g / kg. Gastrointestinal administration was administered once a day for 7 consecutive days. One hour after the last administration, except the normal control group, the other mice were intraperitoneally injected with 0.1% carbon tetrachloride 0.1ml / 10g to create a mouse m...
Embodiment 1
[0101] The capsule preparation of embodiment 1 medicine of the present invention
[0102] 1. Prepare raw materials (kg) according to the following weight ratio:
[0103] Bear bile powder 1, Salvia miltiorrhiza 20, Turmeric 40, Astragalus 60
[0104] 2. Preparation of Astragalus dry extract
[0105] 2-1) Add Astragalus membranaceus into water, heat and decoct for extraction, extract twice in total, combine the two extracts of Astragalus membranaceus, wherein the ratio of the volume of water added in the first extraction to the weight of Astragalus membranaceus is 10:1 , that is, add 10L water to 1kg Astragalus membranaceus, the ratio of the volume of water added during the second extraction to the weight of Astragalus membranaceus is 8:1, that is, add 8L water to 1kg Astragalus membranaceus, and the extraction time is 2h / time;
[0106]2-2) Concentrate the extract of Astragalus under reduced pressure to obtain a concentrated extract of Astragalus, wherein the volume of the con...
Embodiment 2
[0117] The granule preparation of embodiment 2 medicine of the present invention
[0118] 1. Prepare raw materials (kg) according to the following weight ratio:
[0119] Bear bile powder 2, Salvia miltiorrhiza 10, Turmeric 30, Astragalus 60
[0120] 2. Preparation of Astragalus dry extract
[0121] 2-1) Add Radix Astragali to ethanol with a concentration of 10% by mass, heat and reflux for extraction, extract 3 times in total, and combine the extracts three times, wherein the ratio of the volume of ethanol to the weight of Radix Astragali during the first extraction It is 10:1, namely 1kg Radix Astragali adds 10L ethanol; The ratio of the volume of ethanol when extracting for the second time and the weight of Radix Astragali is 8:1, namely 1kg Radix Astragali adds 8L ethanol; The weight ratio is 8:1, that is, 1kg of astragalus is added with 8L of ethanol; the extraction time is 1.5h / time;
[0122] 2-2) Concentrate the extract to obtain a concentrated extract of Astragalus, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com