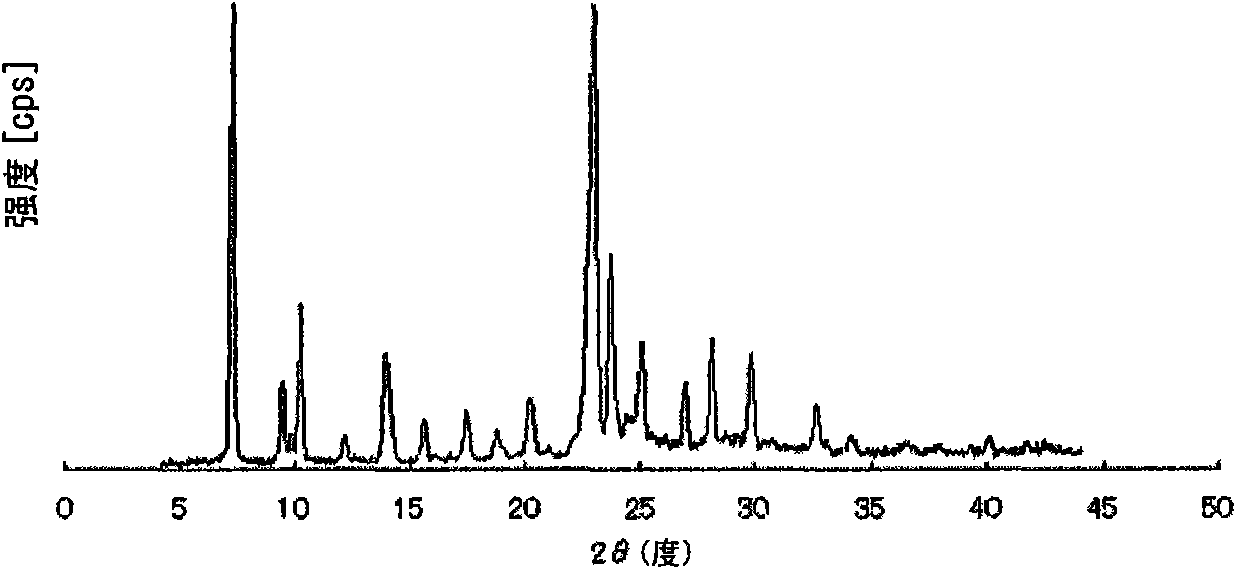
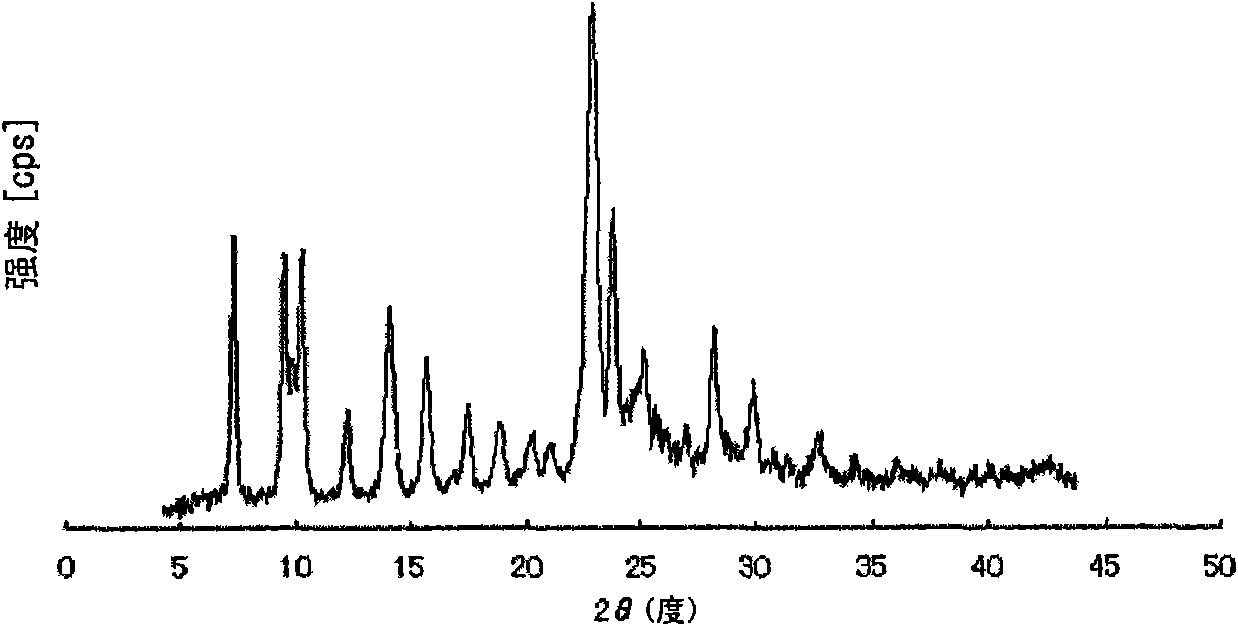
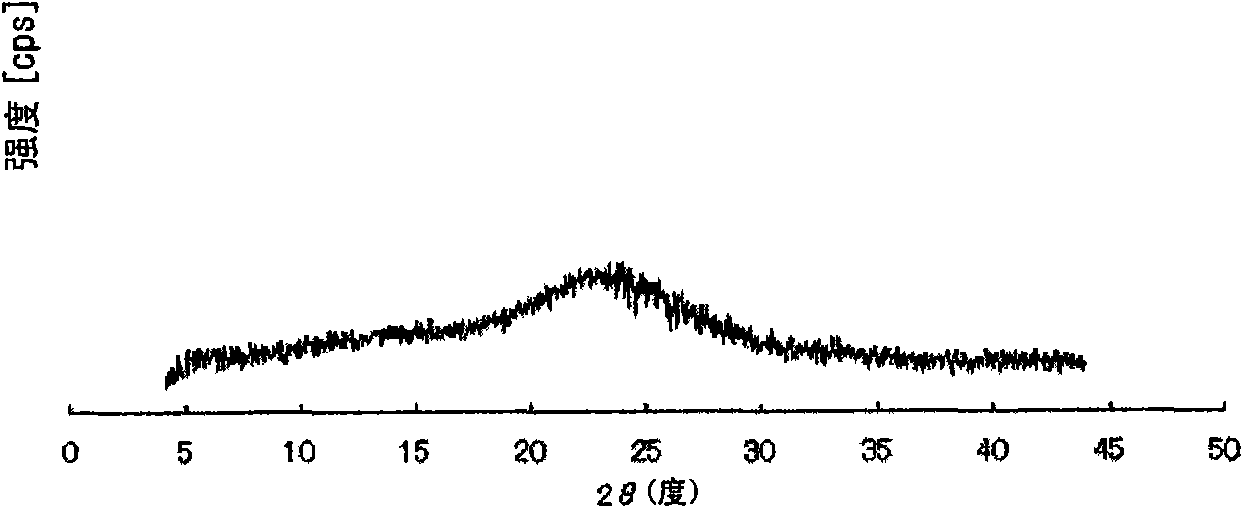
Method for producing pulverized organic compound particle
A technology of organic compounds and manufacturing methods, applied in the direction of active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of inability to manufacture suspended stable microparticles, and inability to obtain small particles , Difficulty removing solvents by finely pulverizing organic compound particles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] In a 0.2L kneader (decomposable kneader, manufactured by YOSHIDA SEISAKUSHO CO., LTD), 9.3 g of indomethacin (melting point: 155-162° C.) with an average particle diameter of 3960 nm, pulverized chlorinated After 140.7 g of sodium (average particle diameter: 5 μm) was uniformly mixed, 35.5 g of glycerol was slowly injected, and the contents were kept in a kneaded powder state, and pulverized at 5° C. for 15 hours. Then, pour the content into 1L of 0.1mol / L aqueous acetic acid solution, and use a homogenizer to disperse evenly, then filter and wash with water, and dry the obtained wet filter cake under reduced pressure at 40°C. 8.7 g of indomethacin having an average particle diameter of 120 nm was obtained.
Embodiment 2
[0187] In a 0.2L kneader, 13.6 g of nifedipine (melting point: 172-175° C.) with an average particle diameter of 4310 nm and 136.4 g of pulverized sodium chloride (with an average particle diameter of 5 μm) were charged and uniformly mixed, 28 g of glycerol was slowly poured, and the content was kept in a kneaded powder form, and pulverized at 10°C for 8 hours. Then, pour the content into 1L of 0.1mol / L aqueous acetic acid solution, and use a homogenizer to disperse evenly, then filter and wash with water, and dry the obtained wet filter cake under reduced pressure at 50°C. 9.8 g of nifedipine with an average particle diameter of 260 nm was obtained.
Embodiment 3
[0191] In a 0.2L kneader, put 9.0 g of cortisone acetate (melting point: about 240° C.) with an average particle diameter of 1060 nm and 141 g of pulverized sodium chloride (average particle diameter of 5 μm) and mix them uniformly. 38 g of glycerin was poured in, and the content was kept in a kneaded powder form, and pulverized at 10° C. for 18 hours. Then, pour the content into 1L of 0.1mol / L aqueous acetic acid solution, and use a homogenizer to disperse evenly, then filter and wash with water, and dry the obtained wet filter cake under reduced pressure at 50°C. 7.4 g of cortisone acetate with an average particle diameter of 260 nm was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com