Targeted therapeutics based on engineered proteins for tyrosine kinases receptors, including igf-ir
An IGF-IR, protein technology, applied in the field of new proteins, can solve the problems of difficult and ineffective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0237] In some embodiments, it may be preferable to glycosylate the proteins of the invention. Preferably, such proteins are fibronectin based scaffolds. Fibronectin-based scaffolds generally do not contain glycosylation sites, however, such glycosylation sites can be engineered into proteins.
[0238] Glycosylation of proteins is typically either N-linked or O-linked. N-linked refers to the attachment of the carbohydrate moiety to the side chain of an asparagine residue. The tripeptide sequences asparagine-X-serine and asparagine-X-threonine (where X is any amino acid except proline) are the recognition sequences for enzymatic attachment of the carbohydrate module to the asparagine side chain . These can be engineered into proteins of the invention, particularly fibronectin-based scaffold proteins and their corresponding polynucleotides. Thus, the presence of either of these two tripeptide sequences in a polypeptide creates a potential glycosylation site. O-linked glycos...
Embodiment 1
[0305] Example 1: Initial characterization of IEG-binding molecules
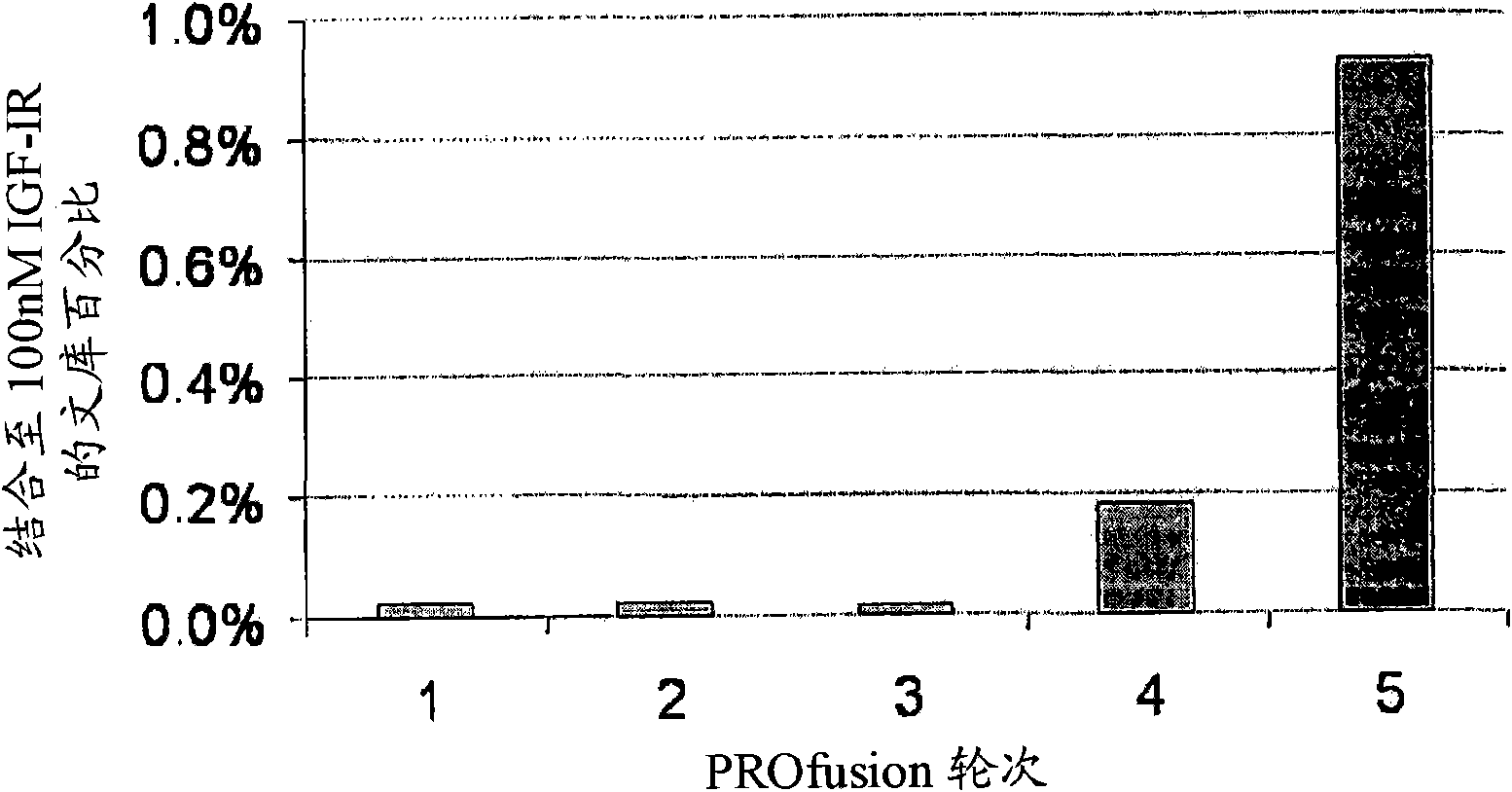
[0306] Based on the scaffold of human fibronectin X-type 3 domains, approximately 10 13 A library of RNA-protein fusion variants (amino acid numbering refers to SEQ ID NO: 1) (tricyclic library; Xu et al., Chemistry & Biology 9:933-942, 2002). After conversion to mRNA / cDNA heteroduplexes, a library of one trillion mRNA / cDNA-protein fusions is bound to 100 nM biotinylated IGF-IR in solution and captured to a streptavidin-coated magnetic beads on. The cDNA is eluted, amplified by PCR, and used to generate a new library of mRNA / cDNA-protein fusions. Five rounds of selection were performed in this manner, and after each round the percentage of bound IGF-IR in the library was monitored by quantitative PCR to identify enrichment for target binding ( figure 1 ).
Embodiment 2
[0307] Example 2: Identification of Competing IGF-IR Clones
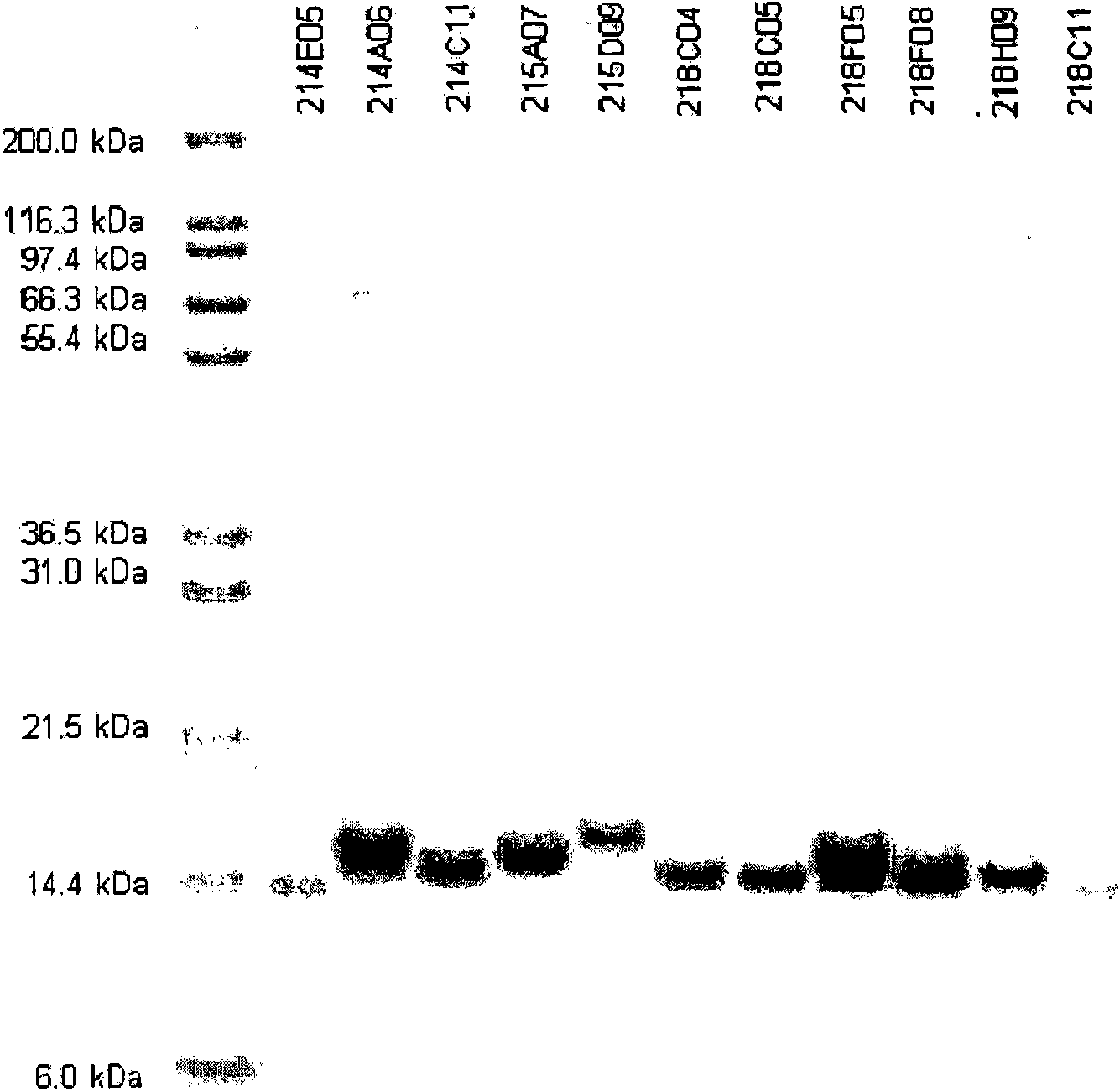
[0308] Proteins encoded by independent clones were analyzed for inhibition of target binding using a competitive binding assay in the presence of IGF-IR. Over one hundred clones were identified as inhibiting IGF-I binding to IGF-IR, suggesting that these clones bind IGF-IR at or near the natural ligand (IGF-I) binding site. The SEQ ID NOs of these one hundred clones (see Figure 30 ) and their percent inhibition in competitive binding assays are presented in Table 1.
[0309]
[0310]
[0311]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com