Sudan hapten and antigen as well as preparation method and application thereof
A reaction and antibody technology, which is applied in peptide preparation methods, chemical instruments and methods, animal/human proteins, etc., can solve the problems of cumbersome operation steps, high cost, and inapplicability to large-scale sample screening and detection, and achieve broad application prospects , high accuracy, and simple sample pretreatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, the preparation of Sudan Red Hapten and Sudan Red Antigen
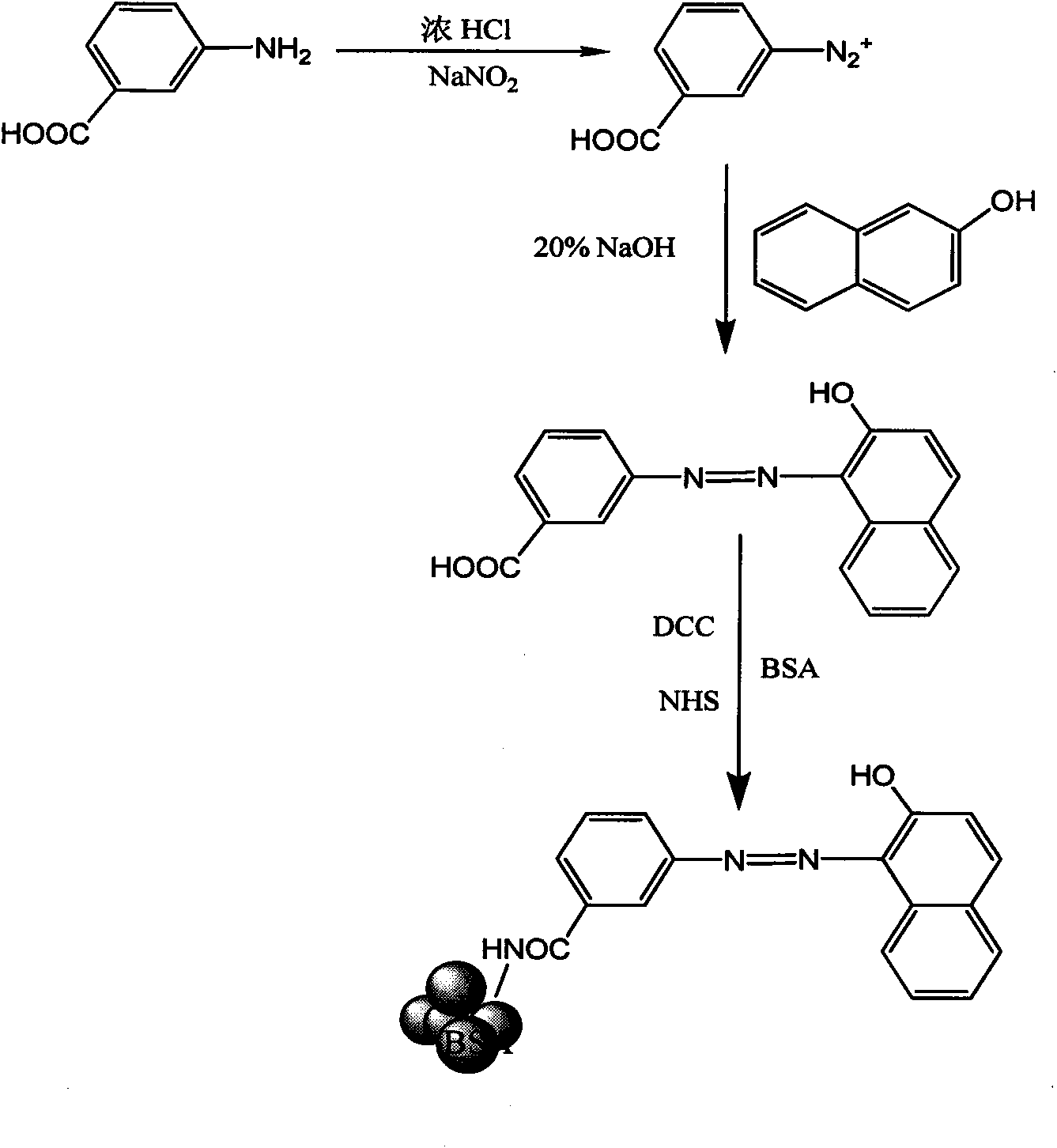
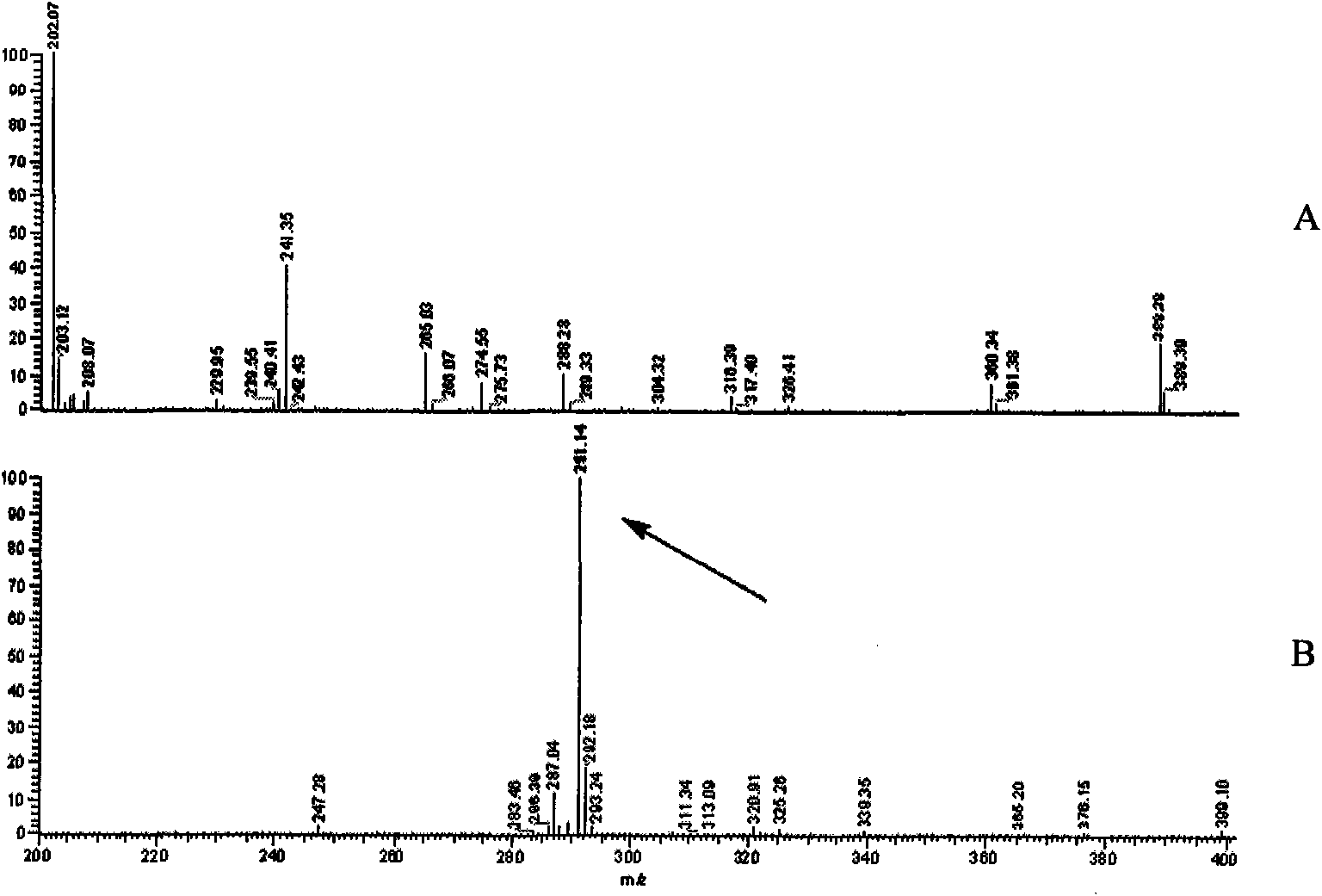
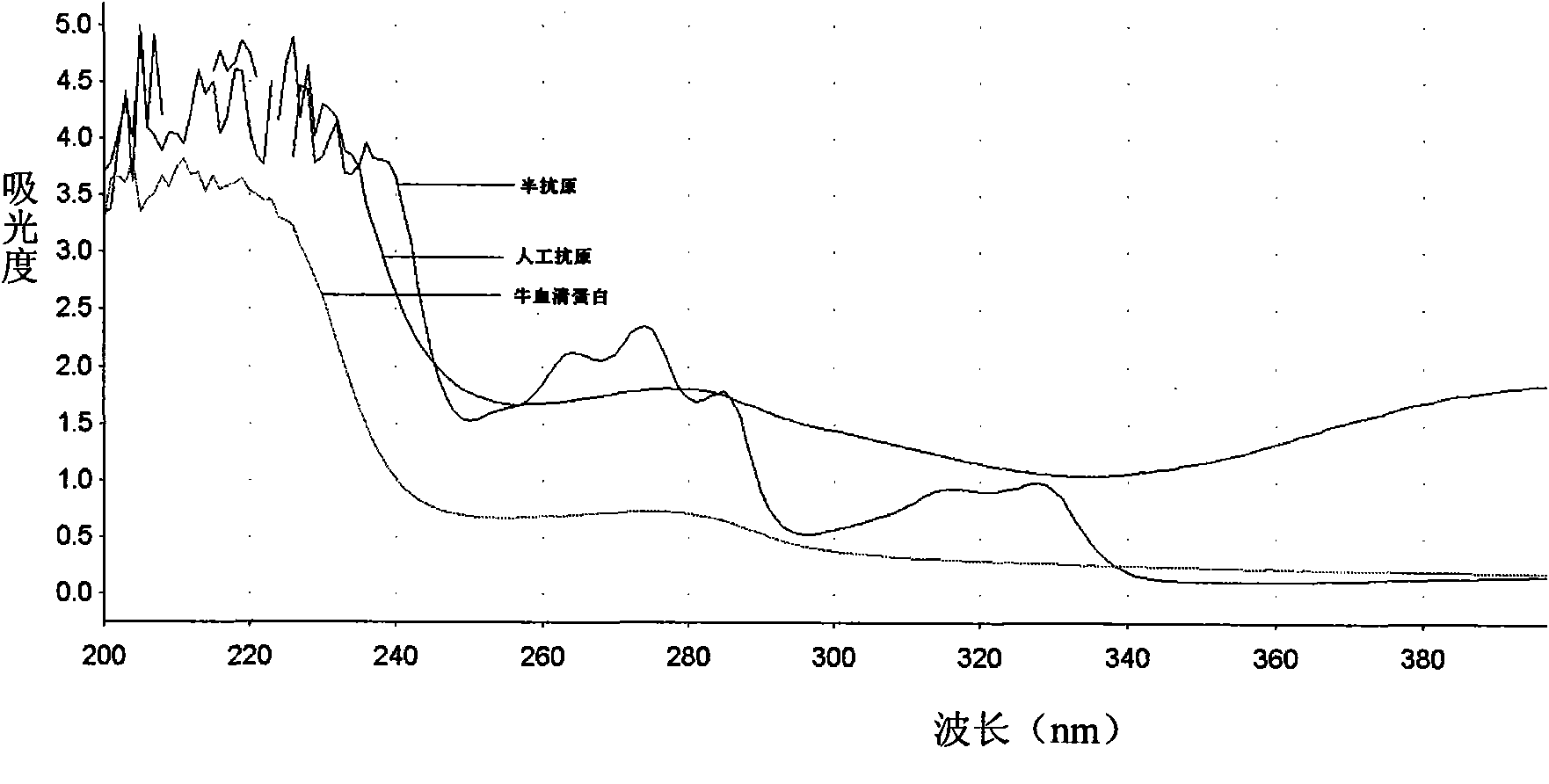
[0042] The preparation process of Sudan red hapten and Sudan red antigen is as follows: figure 1 shown.
[0043] 1. Synthesis of Sudan Red Hapten
[0044] (1) Synthesis
[0045] 1. Diazotization reaction: Dissolve m-aminobenzoic acid in concentrated hydrochloric acid, add an aqueous solution containing sodium nitrite at 5°C, stir and react for 28 minutes to obtain a light yellow diazonium salt solution, which is represented by formula III A solution of the diazide ion of m-aminobenzoic acid.
[0046] The molar ratio of m-aminobenzoic acid, concentrated hydrochloric acid and sodium nitrite is 1:2:1.
[0047]
[0048]2. Coupling reaction: 2-naphthol is dissolved in 20% (mass percentage composition) sodium hydroxide (the amount ratio of 2-naphthol and the feeding material of formula III diazonium ion is 1: 1), is cooled to At 5°C, slowly add m-aminobenzoic acid diazide ion solution, and reac...
Embodiment 2
[0057] Embodiment 2, the preparation of Sudan Red Hapten and Sudan Red Antigen
[0058] The preparation process of Sudan red hapten and Sudan red antigen is as follows: figure 1 shown.
[0059] 1. Synthesis of Sudan Red Hapten
[0060] (1) Synthesis
[0061] 1. Diazotization reaction: Dissolve m-aminobenzoic acid in concentrated hydrochloric acid, add an aqueous solution containing sodium nitrite at 8°C, and stir for 25 minutes to obtain a light yellow diazonium salt solution, which is represented by formula III A solution of the diazide ion of m-aminobenzoic acid.
[0062] The molar ratio of m-aminobenzoic acid, concentrated hydrochloric acid and sodium nitrite is 1:1.5:2.5.
[0063]
[0064] 2. Coupling reaction: 2-naphthol is dissolved in 20% (mass percentage composition) sodium hydroxide (the ratio of the feed material of 2-naphthol and formula III diazonium ion is 1: 1.2), is cooled to Slowly add m-aminobenzoic acid diazide ion solution at 5°C, and react at 8°C f...
Embodiment 3
[0072] Embodiment 3, the preparation of Sudan red hapten and Sudan red antigen
[0073] The preparation process of Sudan red hapten and Sudan red antigen is as follows: figure 1 shown.
[0074] 1. Synthesis of Sudan Red Hapten
[0075] (1) Synthesis
[0076] 1. Diazotization reaction: Dissolve m-aminobenzoic acid in concentrated hydrochloric acid, add an aqueous solution containing sodium nitrite at 10°C, and stir for 30 minutes to obtain a light yellow diazonium salt solution, which is represented by formula III A solution of the diazide ion of m-aminobenzoic acid.
[0077] The molar ratio of m-aminobenzoic acid, concentrated hydrochloric acid and sodium nitrite is 1.5:1:3.
[0078]
[0079] 2. Coupling reaction: 2-naphthol is dissolved in 20% (mass percentage composition) sodium hydroxide (the molar ratio of 2-naphthol and the feeding material of formula III diazonium ion is 1: 1.5), is cooled to 5°C, slowly add m-aminobenzoic acid diazide ion solution, and react at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com