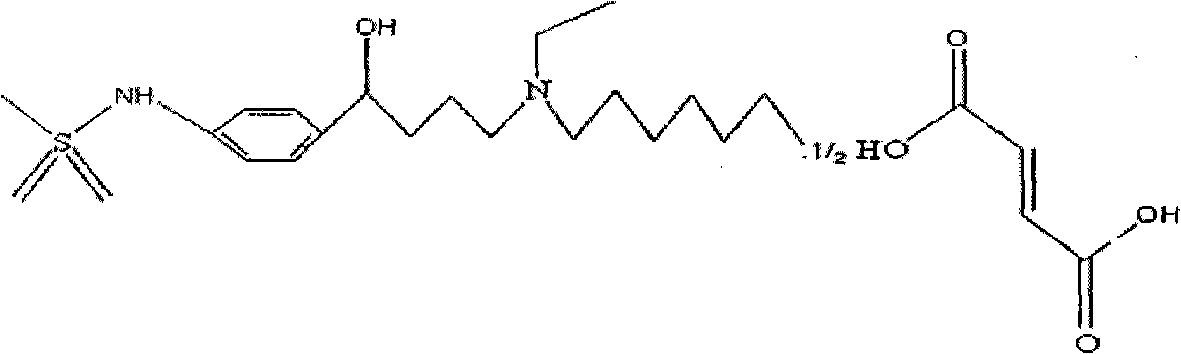
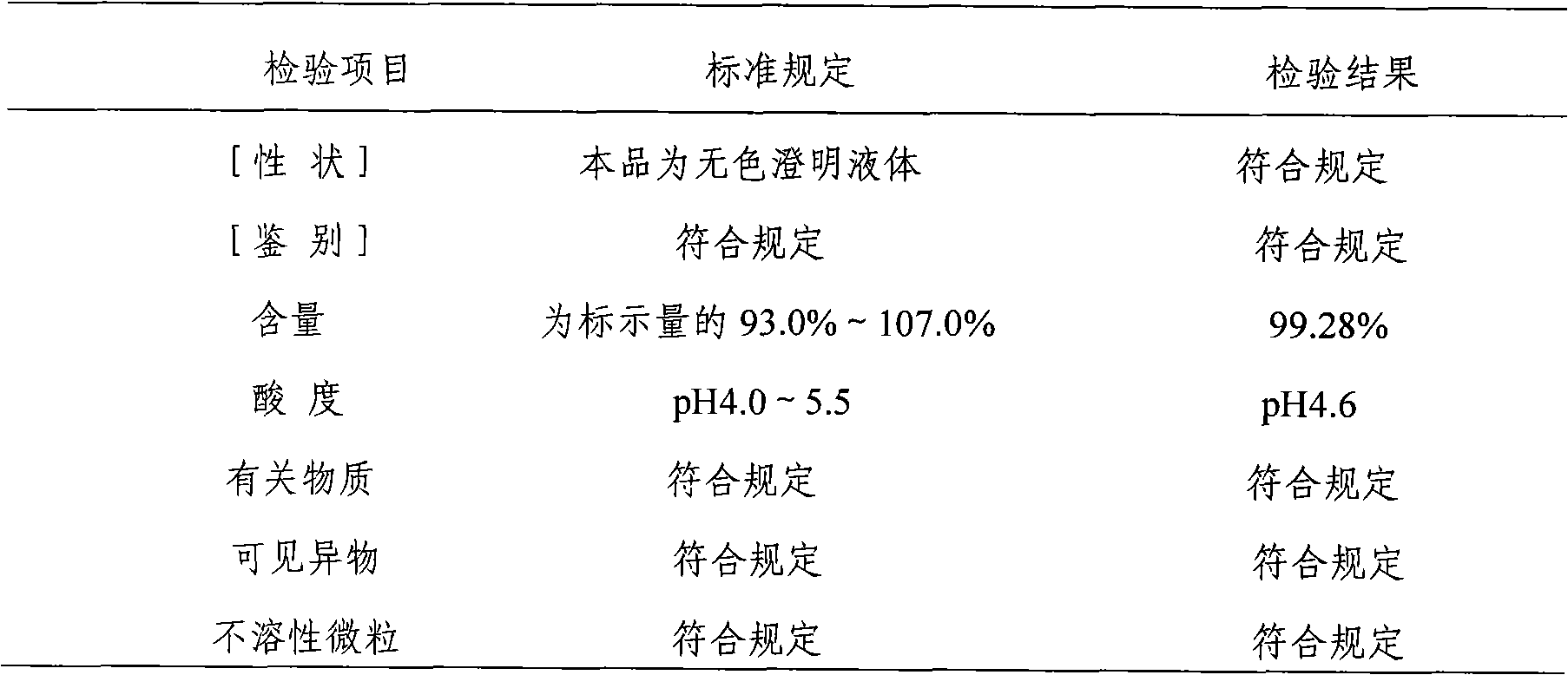
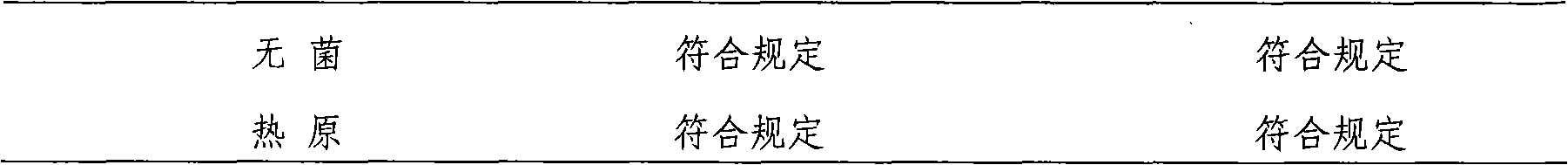
Ibutilide fumarate injection and preparation method thereof
A technology of ibutilide fumarate and injection, which is applied in the field of injection and can solve problems such as poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The ibutilide fumarate injection of the present invention is prepared from the following components: 1000 mg of ibutilide fumarate; 2000 mg of sodium bicarbonate; 5000 mg of sodium bisulfite; 80000 mg of sodium chloride; add water for injection to 10000 ml.
[0040] Its preparation method is as follows:
[0041] 1) Solvent pretreatment: put about 5,000 ml of water for injection into the No. 1 preparation tank, cool to room temperature, and inject CO 2 About 15 to 30 minutes to saturation, measure the pH value, about 6.0.
[0042] 2) Injection preparation: ①Weigh the prescribed amount of sodium bicarbonate, sodium bisulfite, sodium chloride plus about 2000 ml of water for injection, stir slightly to dissolve, and obtain the auxiliary material solution; ②Weigh the prescribed amount of yi fumarate Bullitt, add about 1000 ml of water for injection, stir to dissolve completely, and obtain the raw material solution; ③Add the auxiliary material solution and the raw material s...
Embodiment 2
[0053] Ibutilide fumarate injection of the present invention is made of following raw materials: 1000 mg of ibutilide fumarate; 10000 mg of sodium bicarbonate; 20000 mg of sodium bisulfite; 70000 mg of sodium chloride; 10000ml.
[0054] Its preparation method is as follows:
[0055] 1) Solvent pretreatment: put about 5,000 ml of water for injection into the No. 1 preparation tank, cool to room temperature, and inject CO 2 About 15 to 30 minutes to saturation, measure the pH value, about 6.0.
[0056] 2) Injection preparation: ①Weigh the prescribed amount of sodium bicarbonate, sodium bisulfite, sodium chloride plus about 2000 ml of water for injection, stir slightly to dissolve, and obtain the auxiliary material solution; ②Weigh the prescribed amount of yi fumarate Bullite, add about 1000 ml of water for injection, stir to dissolve completely, and obtain the raw material solution; ③ add the auxiliary material solution and the raw material solution to the No. Evenly, adjust ...
Embodiment 3
[0066] Ibutilide fumarate injection of the present invention is made of following raw materials: 1000 mg of ibutilide fumarate; 5000 mg of sodium bicarbonate; 10000 mg of sodium bisulfite; 75000 mg of sodium chloride; 10000ml.
[0067] Its preparation method is as follows:
[0068] 1) Solvent pretreatment: put about 5000 ml of water for injection into the concentrated preparation tank, cool to room temperature, and inject CO 2 About 15 to 30 minutes to saturation, measure the pH value, about 6.0.
[0069] 2) Injection preparation: ①Weigh the prescribed amount of sodium bicarbonate, sodium bisulfite, sodium chloride plus about 1000 ml of water for injection, stir slightly to dissolve, and obtain the auxiliary material solution; ②Weigh the prescribed amount of idyl fumarate Bullite, add about 1000 ml of water for injection, stir to dissolve completely, and obtain the raw material solution; ③ Add the auxiliary material solution and the raw material solution to the No. 1 prepara...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com