Method for preparing 2-alkyl carboxylic acid
A technology of alkyl carboxylic acid and alkyl group, applied in the field of preparing 2-alkyl carboxylic acid, can solve the problems of expensive raw materials, high cost and rare, and achieve the effects of convenient operation, less by-products and high production process yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
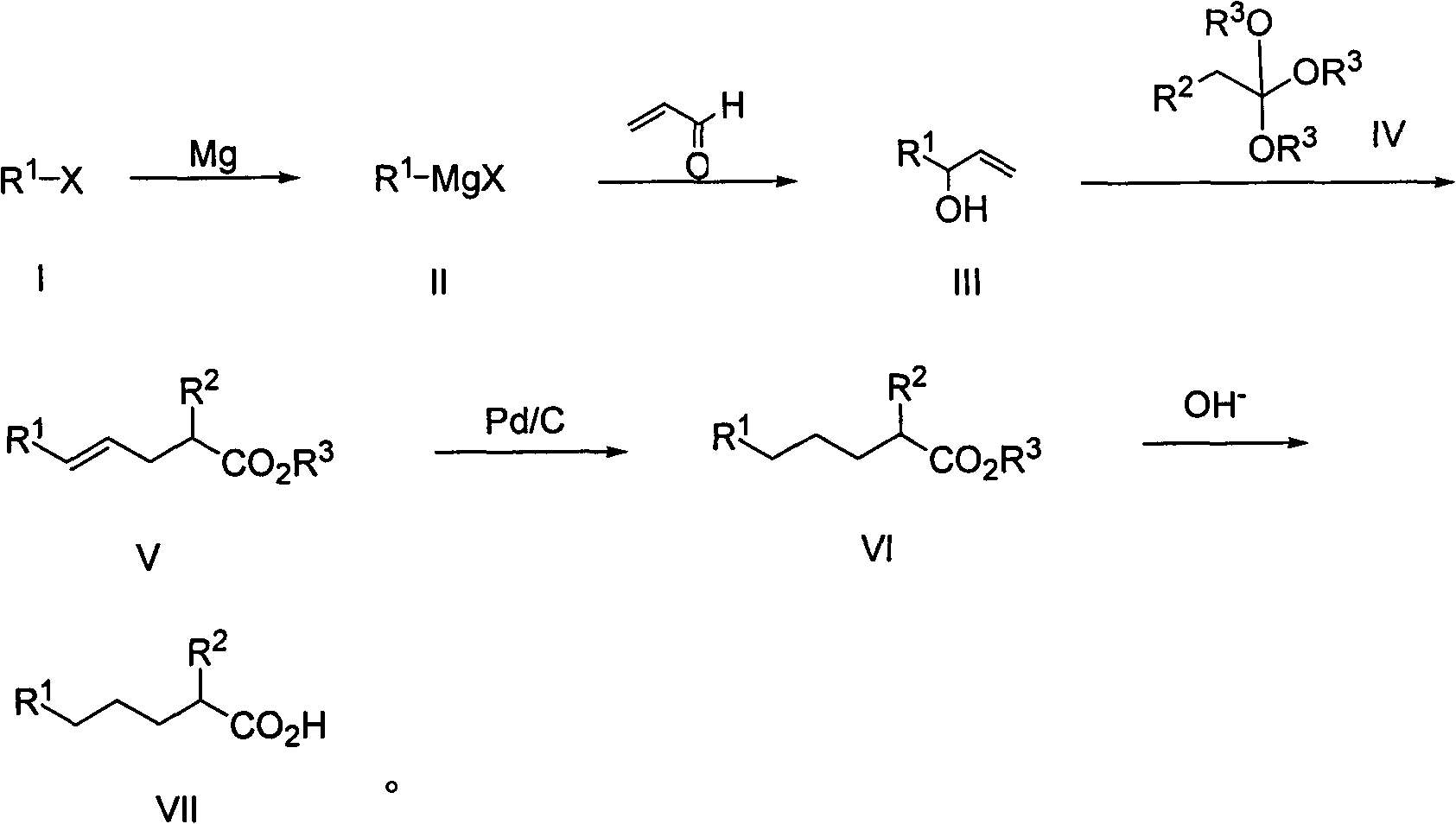
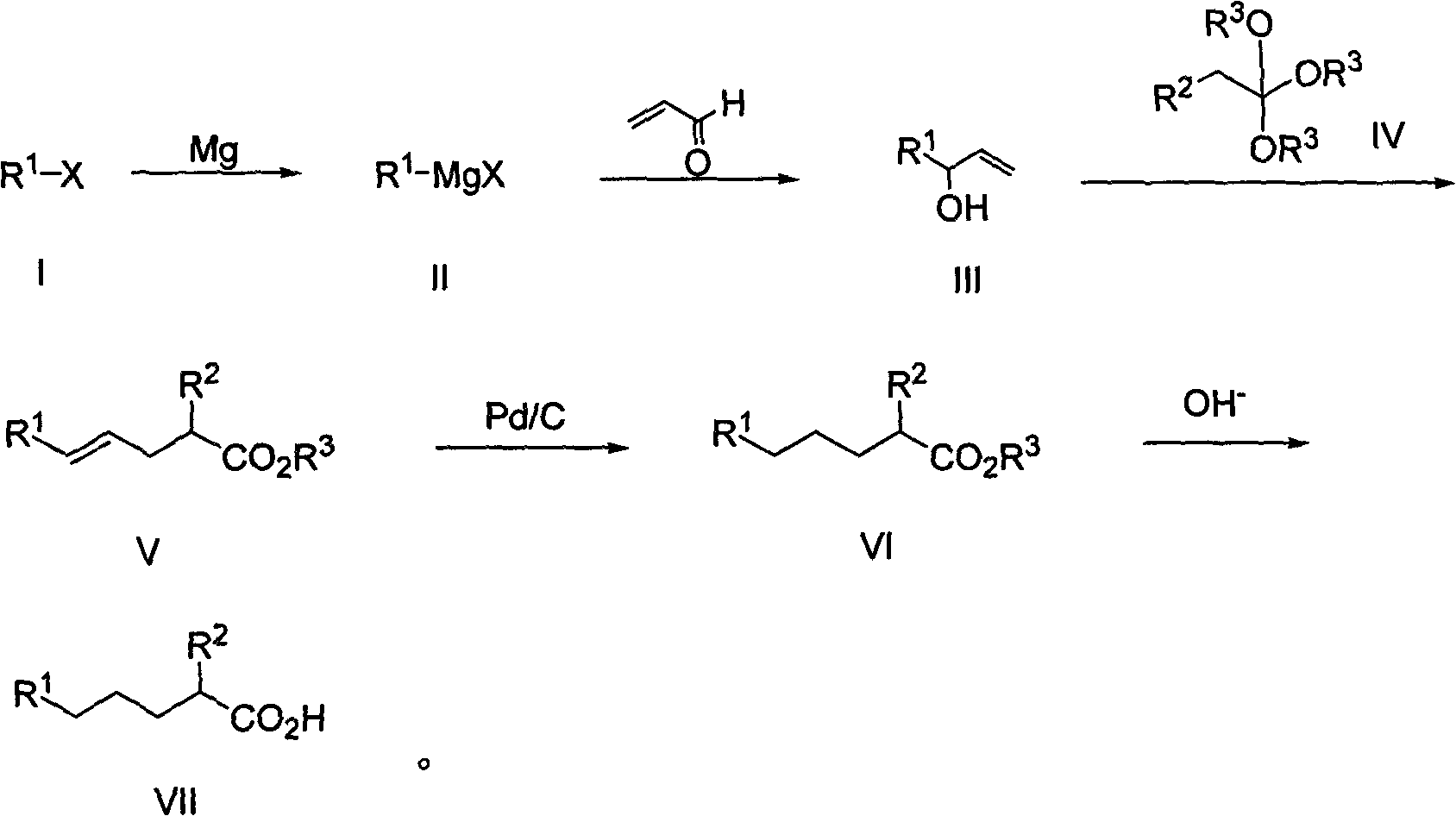
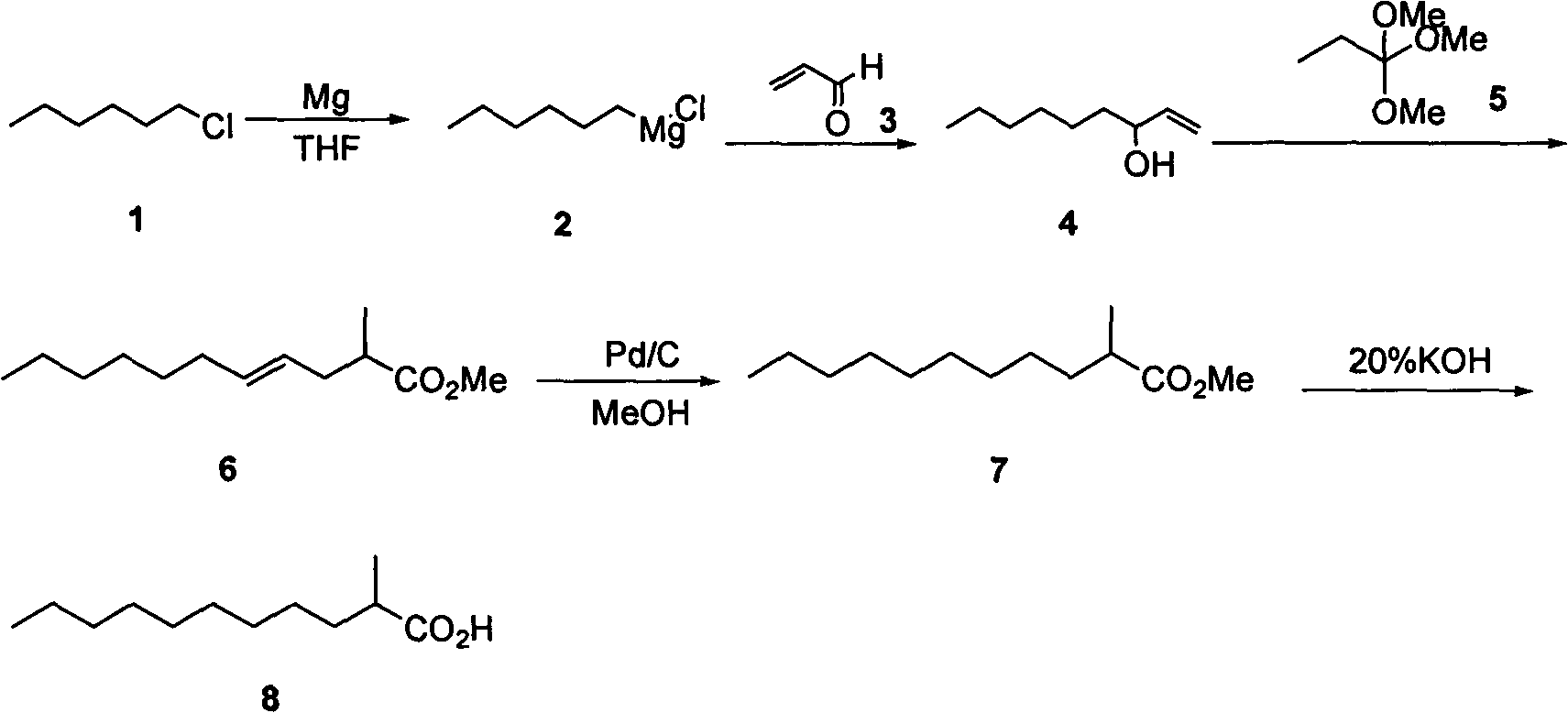
[0027] A kind of method for preparing 2-alkyl carboxylic acid, when R 1 is n-hexyl, R 2 is methyl, R 3 When being a methyl group, the general reaction formula is as follows:
[0028]
[0029] The preparation method comprises the following steps:
[0030] The first step: the synthesis of 3-hydroxyl-1-nonene (4):
[0031] Add 3g (0.125mol) of magnesium chips to a 250ml four-necked bottle, add 20ml of tetrahydrofuran (THF), stir under nitrogen protection, add a small grain of iodine (40mg) and 0.5ml of 1,2-dibromoethane as the initiator, heat, System initiation, reaction at 60°C, 13.7 g (0.113 mol) of n-chlorohexane (1) was slowly added dropwise, and the addition was completed in 50 minutes, and the reaction was carried out at 45°C. After 2 hours, GC tracking showed that the reaction of raw materials was complete. Transfer the reaction solution into a 250ml dropping funnel, slowly dropwise add acrolein (3) 9.5g, (0.170mol) and THF (50ml) mixture, control the temperature at...
Embodiment 2
[0054] A kind of method for preparing 2-alkyl carboxylic acid, when R 1 is n-propyl, R 2 is ethyl, R 3 When being a methyl group, the general reaction formula is as follows:
[0055]
[0056] The preparation method comprises the following steps:
[0057] The first step: the synthesis of 3-hydroxyl-1-hexene (11):
[0058] Add 5g (0.208mol) magnesium chips to a 250ml four-necked bottle, add 30ml THF, stir under nitrogen protection, add a small grain of iodine (about 40mg) and 0.5ml of 1,2-dibromoethane as the initiator, heat, and the system initiates , reacted at 60°C, slowly added 14.9g (0.189mol) of n-chloropropane (9) dropwise, and the addition was completed in 50 minutes. After reacting at 40°C for 2 hours, GC tracking showed that the reaction of the raw materials was completed. The reaction solution was slowly added dropwise to a mixture of 15.9 g (0.283 mol) of acrolein (3) and THF (50 ml), the temperature was controlled at 5-10°C, the dropwise addition was complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com