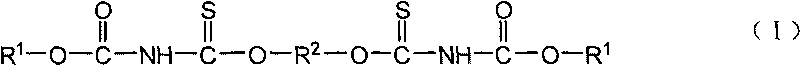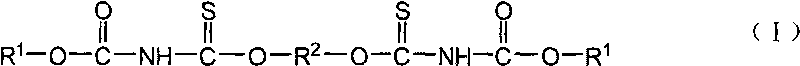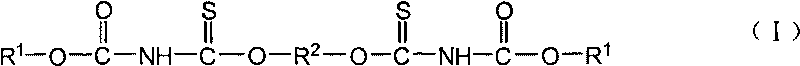Sulfide flotation collector and preparation method
A technology of sulfide ore and collector, which is applied in flotation, solid separation, etc., can solve the problems of poor adaptability, achieve weak collection capacity, good selectivity, and improve the recovery rate of flotation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1N, the preparation of N'-diethoxycarbonyl-O, O'-(1,2-ethylene) dithiocarbamate
[0024] 31 parts of 1,2-ethanediol plus acetone are configured into an ethylene glycol-acetone solution with a mass concentration of 30%, and slowly added to 131.2 parts of N-ethoxycarbonyl isothiocyanates in a three-necked flask. After the dropwise addition, the organic mixture continued to react at 45-50° C. for 4 hours, and the reaction was completed. After the solvent acetone was distilled off under reduced pressure, 158 parts of yellow product were obtained, which contained 143 parts of N, N'-diethoxycarbonyl-O, O'-(1,2-ethylene) dithiocarbamate, and the product purity was 90.5 %.
Embodiment 2
[0025] Embodiment 2N, the preparation of N'-diethoxyalkyl-O, O'-(1,4-butylene) dithiocarbamate
[0026] 45 parts of 1,4-butanediol plus acetone are configured into a butanediol-acetone solution with a mass concentration of 20%, and slowly added to 131.2 parts of N-ethoxycarbonyl isothiocyanates in a three-necked flask. After the dropwise addition, the organic mixture continued to react at 45-50° C. for 4 hours, and the reaction was completed. After the solvent acetone was distilled off under reduced pressure, 171 parts of yellow product were obtained, which contained 157 parts of N, N'-diethoxyalkyl-O, O'-(1,4-butylene) dithiocarbamate, and the product purity was 91.8% .
Embodiment 3
[0027] Embodiment 3N, the preparation of N'-dibutoxyalkyl-O, O'-(1,4-butylene) dithiocarbamate
[0028] Add 45 parts of 1,4-butanediol and tetrahydrofuran to form a butanediol-tetrahydrofuran solution with a mass concentration of 20%, and slowly add it to 159.3 parts of N-butoxycarbonyl isothiocyanates in a three-necked flask. After the dropwise addition, the organic mixture continued to react at 45-50° C. for 4 hours, and the reaction was completed. After the solvent tetrahydrofuran was distilled off under reduced pressure, 195 parts of yellow product were obtained, which contained 176 parts of N, N'-dibutoxyalkyl-O, O'-(1,4-butylene) dithiocarbamate, and the product purity was 90.3% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com