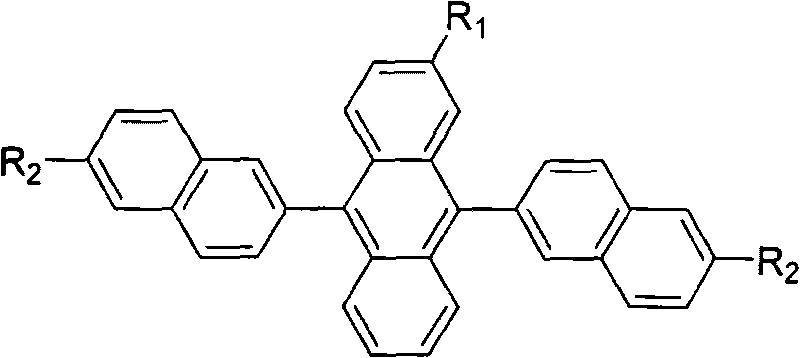
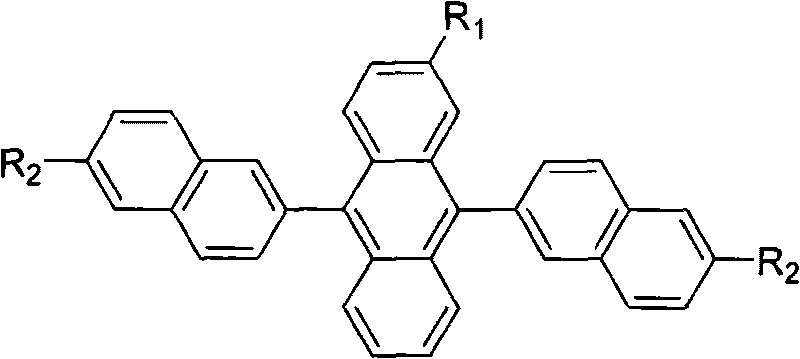
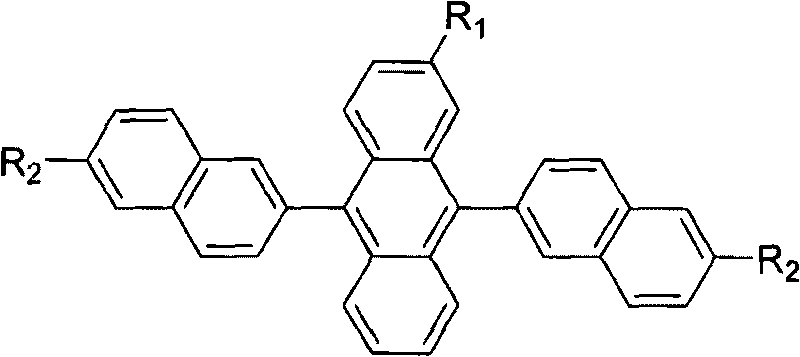
Substituted 9,10-dinaphthyl anthracene blue light-emitting organic electro-luminescent material and method for preparing same
A luminescent, 10-technology, applied in luminescent materials, hydrocarbon production from oxygen-containing organic compounds, organic chemistry, etc., can solve the problems of low fluorescence quantum efficiency, easy crystallization, and difficulty in forming amorphous films, etc., to achieve Effects of increased material efficiency, increased spacing, and resolution of thin film morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0025] R 1 is methyl, R 2 Synthesis route of 6-tert-butyl-2-naphthyl substituted anthracene blue-light organic electroluminescent material for tert-butyl group:
[0026]
[0027] (1) Synthesis of 2-methyl-9,10-bis(6-tert-butyl-2-naphthalene-)-9,10-diol
[0028] Add 6-tert-butyl-2-bromonaphthalene (15.79 g, 0.06 mol) and THF (60 ml) into a four-necked round-bottomed flask connected with a constant pressure dropping funnel and a thermometer, stir, and protect with nitrogen. Cool down in a dry ice acetone bath, and after the reaction solution cools down to -78°C, start adding the calculated amount of n-butyllithium / n-hexane solution dropwise, control the dropping rate, and keep the temperature of the reaction solution below -70°C. The reaction solution gradually turned yellow and turbid. After dropping, stir for 0.5 hr and set aside. Add 2-methylanthraquinone (5.56g, 0.027mol) and THF (90ml) into another three-necked flask, stir, protect with nitrogen, and cool down to -78...
Embodiment 12
[0036] (1) Synthesis of 2-methyl-9,10-bis(6-tert-butyl-2-naphthalene-)-9,10-diol
[0037] Other conditions remain unchanged, change 6-tert-butyl-2-bromonaphthalene to 0.04mol, THF to 40ml, 2-methylanthraquinone to 0.022mol, THF that appears later to 75ml, and ethyl acetate to 150ml, 7.4g of light yellow solid was obtained, the yield was 68%.
[0038] (2) Synthesis of 9,10-bis(6-tert-butyl)naphthalene-2-methylanthracene
[0039] Other conditions are unchanged, tin protochloride dihydrate is changed into 0.03mol, 2-methyl-9,10-bis(6-tert-butyl-2-naphthalene-)-9,10-diol is changed into 0.03mol, 10.12 g of white powder was obtained with a yield of 77%.
[0040] (3) Spectral properties of 9,10-di(6-tert-butyl)naphthalene-2-methylanthracene
[0041] With embodiment 1.1.
Embodiment 2
[0043] R 1 is ethyl, R 2 Synthetic route of 6-tert-butyl-2-naphthyl substituted anthracene-based blue-light organic electroluminescent material that is tert-butyl:
[0044]
[0045] (1) Synthesis of 2-ethyl-9,10-bis(6-tert-butyl-2-naphthalene-)-9,10-diol
[0046] In a four-neck round bottom flask connected with a constant pressure dropping funnel and a thermometer, add (15.79 g, 0.06 mol) 6-tert-butyl-2-bromonaphthalene, THF (60 mL), stir, and protect with nitrogen. Cool down in a dry ice acetone bath, and after the reaction solution cools down to -78°C, start adding the calculated amount of n-butyllithium / n-hexane solution dropwise, control the dropping rate, and keep the temperature of the reaction solution below -70°C. The reaction solution gradually turned yellow and turbid. After dropping, stir for 0.5 hr and set aside. Add 2-ethylanthraquinone (5.56 g, 0.027 mol) and THF (90 mL) into another three-necked flask, stir, protect with nitrogen, and cool down to -78° C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com