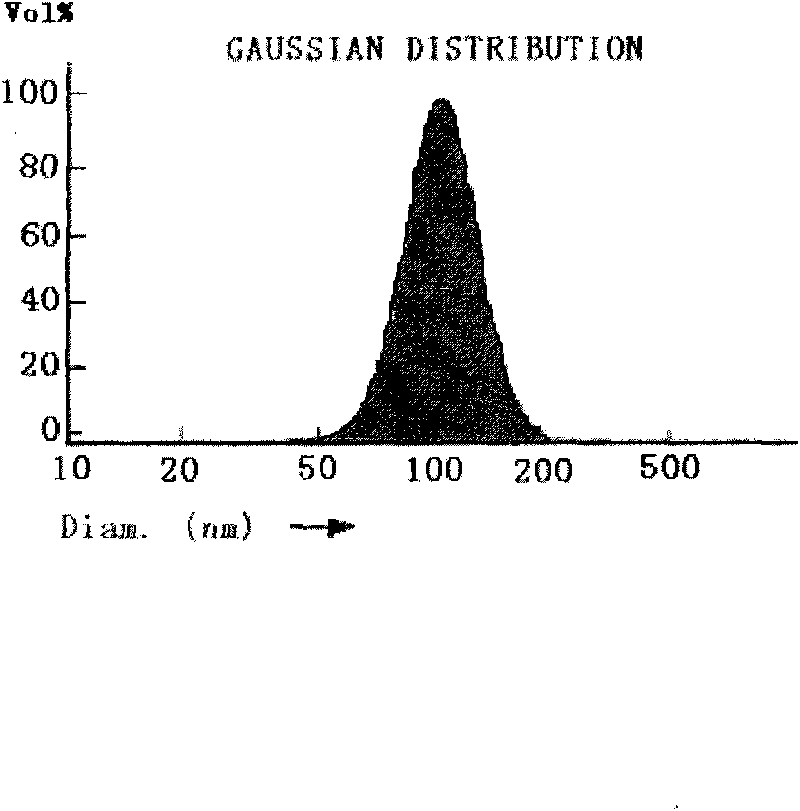
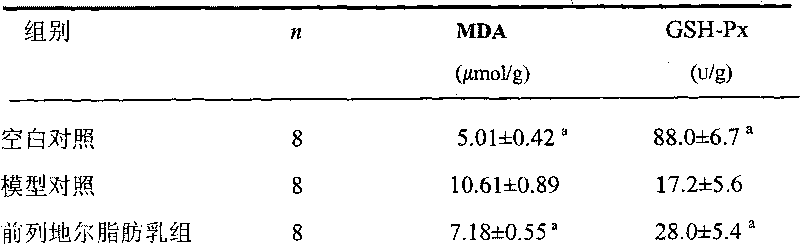
Prostaglandin E1 long-circulation fat microsphere preparation for intravenous injection and preparation method thereof
A technology of alprostadil and lipid microspheres, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, oil/fat/wax non-active ingredients, etc. It can solve the problem that the treatment effect of alprostadil is not very satisfying to patients, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1 The most preferred alprostadil lipid microsphere preparation of the present invention
[0099] Alprostadil 5.0mg
[0100] Soybean oil (for injection) 100.0g
[0101] Lecithin (for injection) 18.0g
[0102] Sodium Oleate 2.4g
[0103] Polyethylene Glycol-Phospholipid 20.0g
[0104] Vitamin E (for injection) 5.0g
[0105] Glycerin 22.1g
[0106] Add water for injection to 1000ml
[0107] Take alprostadil, soybean oil, add lecithin, vitamin E, polyethylene glycol-phospholipid (PEG-DSPE), under nitrogen protection, heat to 75 ℃, stir for about 10min to make the various lipid compounds added Fully dissolve, dissolve and mix. Another take 800ml water for injection, add glycerol and sodium oleate. Under nitrogen protection, the oil solution containing the drug was added to the aqueous solution of glycerol and sodium oleate under shear and stirring conditions to prepare colostrum, and the total amount was adjusted to 1000ml. Homogenize 5-8 times with a hig...
example 2
[0108] Example 2 Long-circulating alprostadil lipid microsphere pharmaceutical formulation with the highest alprostadil content (only soybean oil is used as an example)
[0109] prescription:
[0110] Alprostadil 1.0g
[0111] Soybean oil (for injection) 100.0g
[0112] Lecithin (for injection) 18.0g
[0113] Sodium Oleate 2.4g
[0114] Polyethylene Glycol-Phospholipid 20.0g
[0115] Vitamin E (for injection) 5.0g
[0116] Glycerin 22.1g
[0117] Add water for injection to 1000ml
[0118] Take 1.0 g of alprostadil and 100.0 g of soybean oil, add 18.0 g of lecithin and 20.0 g of polyethylene glycol-phospholipid (PEG-DSPE), under nitrogen protection, heat to 75 ° C, and stir for about 10 minutes to make the added Various lipid compounds are fully dissolved, then 5.0 g of vitamin E is added, dissolved and mixed. Another take 800ml of water for injection, add 22.1g of glycerol and 2.4g of sodium oleate. Under nitrogen protection, the oil solution containing the drug was a...
example 3
[0120] Example 3 Long-circulating alprostadil lipid microsphere pharmaceutical formulation with minimal polyethylene glycol-phospholipid content (only soybean oil is used as an example)
[0121] prescription:
[0122] Alprostadil 5.0mg
[0123] Soybean oil (for injection) 100.0g
[0124] Lecithin (for injection) 18.0g
[0125] Sodium Oleate 2.4g
[0126] Polyethylene Glycol-Phospholipid 2.0g
[0127] Vitamin E (for injection) 5.0g
[0128] Glycerin 22.1g
[0129] Add water for injection to 1000ml
[0130] Take 5.0 mg of alprostadil, 100.0 g of soybean oil, add 18.0 g of lecithin and 2.0 g of polyethylene glycol-phospholipid (PEG-DSPE), under nitrogen protection, heat to 75 ° C, and stir for about 10 min to make the added Various lipid compounds are fully dissolved, then 5.0 g of vitamin E is added, dissolved and mixed. Take another 800ml of water for injection and add 22.1g of glycerol. Under nitrogen protection, the oil solution containing the drug was added to the g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com