Method for preparing arrayed organic nanoparticles
A technology of nanoparticles and organic molecules, applied in nanotechnology, nanotechnology, nanostructure manufacturing, etc., to achieve the effect of improving crystallization performance, simple operation, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) 972mg (1.43mmol) tetraphenyl zinc porphyrin (ZnTPP) was dissolved in 20ml methylene chloride; 203mg (0.80mmol) I 2 Dissolved in 10ml of dichloromethane; 332mg (1.60mmol) AgClO 4 Dissolved in 3ml of acetonitrile; 2 solution of dichloromethane and AgClO 4 The acetonitrile solution of ZnTPP is added in the dichloromethane solution of ZnTPP, and the gained precipitate is washed and dried with petroleum ether and dissolved in acetonitrile to obtain a cationic free radical solution of 1mmol / L ZnTPP;
[0027] The reaction formula is: ZnTPP+1 / 2I 2 +AgClO 4 →AgI+ZnTPP + ·ClO 4 -
[0028] 2) Under sealed and closed light conditions, the arrayed silver electrode substrate was immersed in the cationic radical solution of ZnTPP for 24 hours, then soaked in acetonitrile and isopropanol for 1 minute, and dried with nitrogen to obtain arrayed ZnTPP organic nanoparticles .
[0029] The reaction formula is: ZnTPP + ·+Ag→ZnTPP+Ag +
[0030] Gained arrayed ZnTPP organic nano...
Embodiment 2
[0032] 1) 720mg (1.43mmol) perylene (Perylene) was dissolved in 20ml methylene chloride; 203mg (0.80mmol) I 2 Dissolved in 10ml of dichloromethane; 332mg (1.60mmol) AgClO 4 Dissolved in 3ml of acetonitrile; 2 solution of dichloromethane and AgClO 4 Add the acetonitrile solution of Perylene into the dichloromethane solution of Perylene and stir for 0.5h to react, centrifuge the obtained precipitate, wash with dichloromethane and dry, add acetonitrile dropwise over the top, seal the container and let it stand for 16 hours, take the supernatant liquid It is a cationic free radical solution of Perylene, and its concentration is 0.1mmol / L as measured by cetyltrimethylammonium bromide (CTAB) solution;
[0033] The reaction formula is: Pe+1 / 2I 2 +AgClO 4 →AgI+Pe + ·ClO 4 -
[0034] 2) Under sealed and closed light conditions, the arrayed silver electrode substrate was immersed in the cationic radical solution of Perylene for 12 hours, then soaked in acetonitrile and isopropan...
Embodiment 3
[0038] 1) Add 1mg (2×10 -6 mol) hexathiophene (Sexithiophene, 6T) was dissolved in 5mL of dichloromethane; 0.8mg (5 × 10 -4 mol) FeCl 3 Dissolve in 5mL dichloromethane; FeCl 3 The dichloromethane solution of 6T is added to the dichloromethane solution of 6T, and the dark blue 10 -6 6T cationic free radical solution of mol / L;
[0039] The reaction formula is: 6T+2FeCl 3 →6T + ·+FeCl 4 - +FeCl 2
[0040]2) Under sealed and closed light conditions, the arrayed silver electrode substrate was immersed in 6T cationic radical solution for 24 hours, then soaked in acetonitrile and isopropanol for 1 minute, and dried with nitrogen to obtain arrayed 6T organic nanoparticles .
[0041] The reaction formula is: 6T + ·+Ag→6T+Ag +
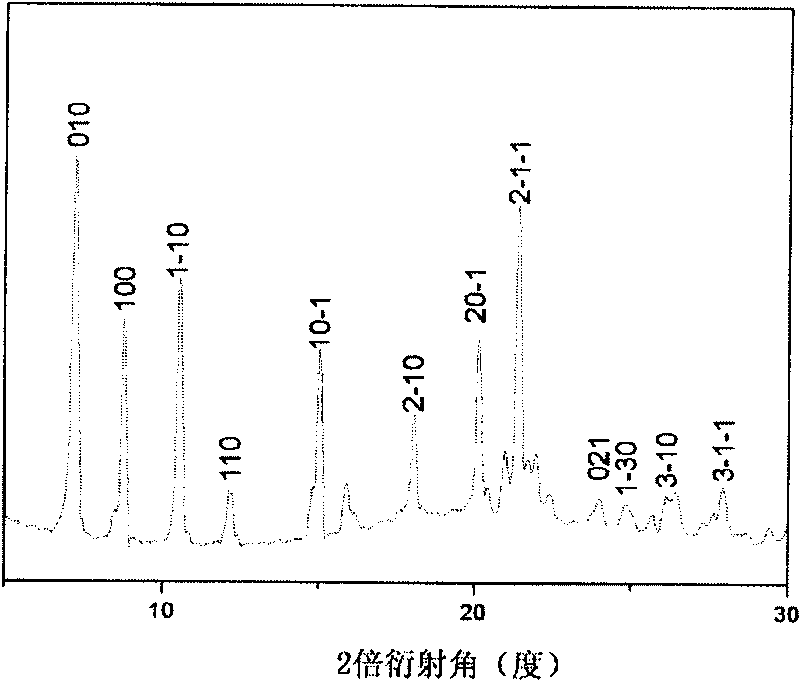
[0042] Gained arrayed 6T organic nanoparticles (number 3 # ) scanning electron microscope images are shown in Figure 5 , the XRD images are shown in Figure 6 middle. It can be seen from the figure that the arrayed organic nanoparticles obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com