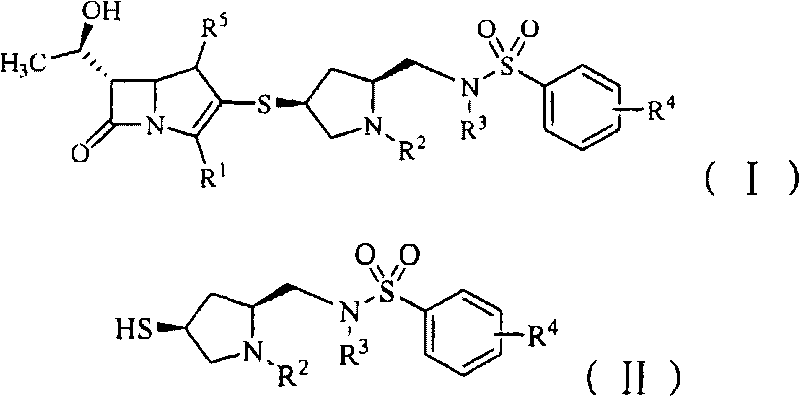
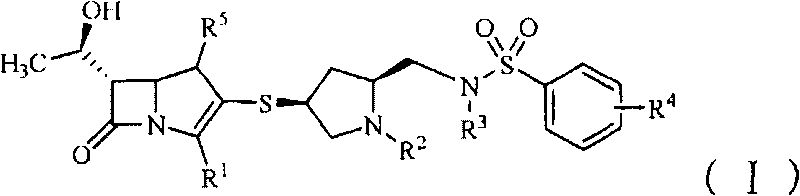
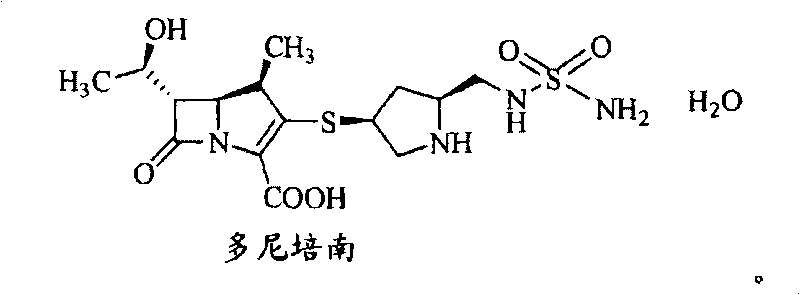
Benzenesulfonamido methylene substituted mercapto pyrrolidine carbapenem derivatives
A technology of substituents and aminosulfonyl groups is applied in the field of mercaptopyrrolidine carbapenem derivatives substituted by benzenesulfonamide methylene, which can solve the problem of low clinical availability, inability to meet clinical needs, and increased bacterial resistance. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] The present invention also provides the preparation method of above-mentioned compound, described method is but not limited to following method, and this method has following reaction equation:
[0098]
[0099] Reaction steps:
[0100] The preparation of compound shown in step 1 formula (a)
[0101] Add raw material 1 and methanol to the dry reaction bottle, mix uniformly, then add thionyl chloride solution dropwise therein, stir the obtained mixture for a period of time, then cool it, and precipitate crystals from the mixture. The obtained crystals are filtered, and the obtained filter cake is dried to obtain the compound represented by formula (a).
[0102] Preparation of compound shown in step 2 formula (b)
[0103] In a dry reaction flask, add the triethylamine solution to the dichloromethane solution of the compound represented by the formula (a) obtained in the previous step, stir the resulting mixture, and then add triethylamine and methanesulfonyl chlor...
Embodiment 1
[0134] Example 1 (4R, 5S, 6S)-3-[(2S, 4S)-2-[4-methyl-benzenesulfonamido]methylene- Pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0] Preparation of hept-2-ene-2-carboxylic acid (i.e. compound 1 of the present invention)
[0135]
[0136] Step 1 Preparation of (2S, 4R)-4-hydroxyl-2-methoxycarbonylpyrrolidine hydrochloride
[0137] In a dry reaction flask, 13.1 g (100 mmol) of trans-4-hydroxy-L-proline (commercially available from Shanghai Qiude Biochemical Co., Ltd.) and 50 ml of methanol were added and mixed uniformly. To the resulting mixture was added dropwise 8 ml of thionyl chloride at 0°C. After the dropwise addition, the resultant was warmed up to room temperature and stirred for 20 min, then warmed up to 40° C. and stirred for 14 h. The resultant was cooled, from which white crystals were precipitated, and a filter cake was obtained by filtration. The resulting filter cake was dried to obtain 13.9 g of the product, with a yiel...
Embodiment 2
[0156] Example 2 (4R, 5S, 6S)-3-[(2S, 4S)-2-[3-methyl-benzenesulfonamido]methylene- Pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0] Preparation of hept-2-ene-2-carboxylic acid (i.e. compound 2 of the present invention)
[0157]
[0158] Step 1 (2S,4S)-4-Mercapto-2-[N-(tert-butoxycarbonyl)-3-methyl-benzenesulfonamido] Preparation of methylene-1-(tert-butoxycarbonyl)pyrrolidine
[0159] With reference to the preparation method of step 5 in Example 1, 5.5 g (20mmol ) and N-(tert-butoxycarbonyl)-3-methyl-benzenesulfonamide (commercially available from Hengzhou Ruier Chemical Co., Ltd.) 9.0g (33mmol) to get (2S, 4S)-4-mercapto-2- [N-(tert-butoxycarbonyl)-3-carboxy-benzenesulfonamido]methylene-1-(tert-butoxycarbonyl)pyrrolidine 5.3 g, yield: 54.6%.
[0160] Step 2 (4R,5S,6S)-3-[(2S,4S)-2-[N-(tert-butoxycarbonyl)-3-methyl-benzenesulfonyl Amino]methylene-1-(tert-butoxycarbonyl)pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4- Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com