Method for synthesizing phyllocnistis citrella stainton pheromone compound
A technology of citrus leaf miner and synthetic method, which is applied in the fields of oxidative preparation of carbonyl compounds, organic chemistry, etc., and can solve problems affecting consumers' health, pathogenic bacteria invasion of citrus canker disease, and excessive pesticide residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
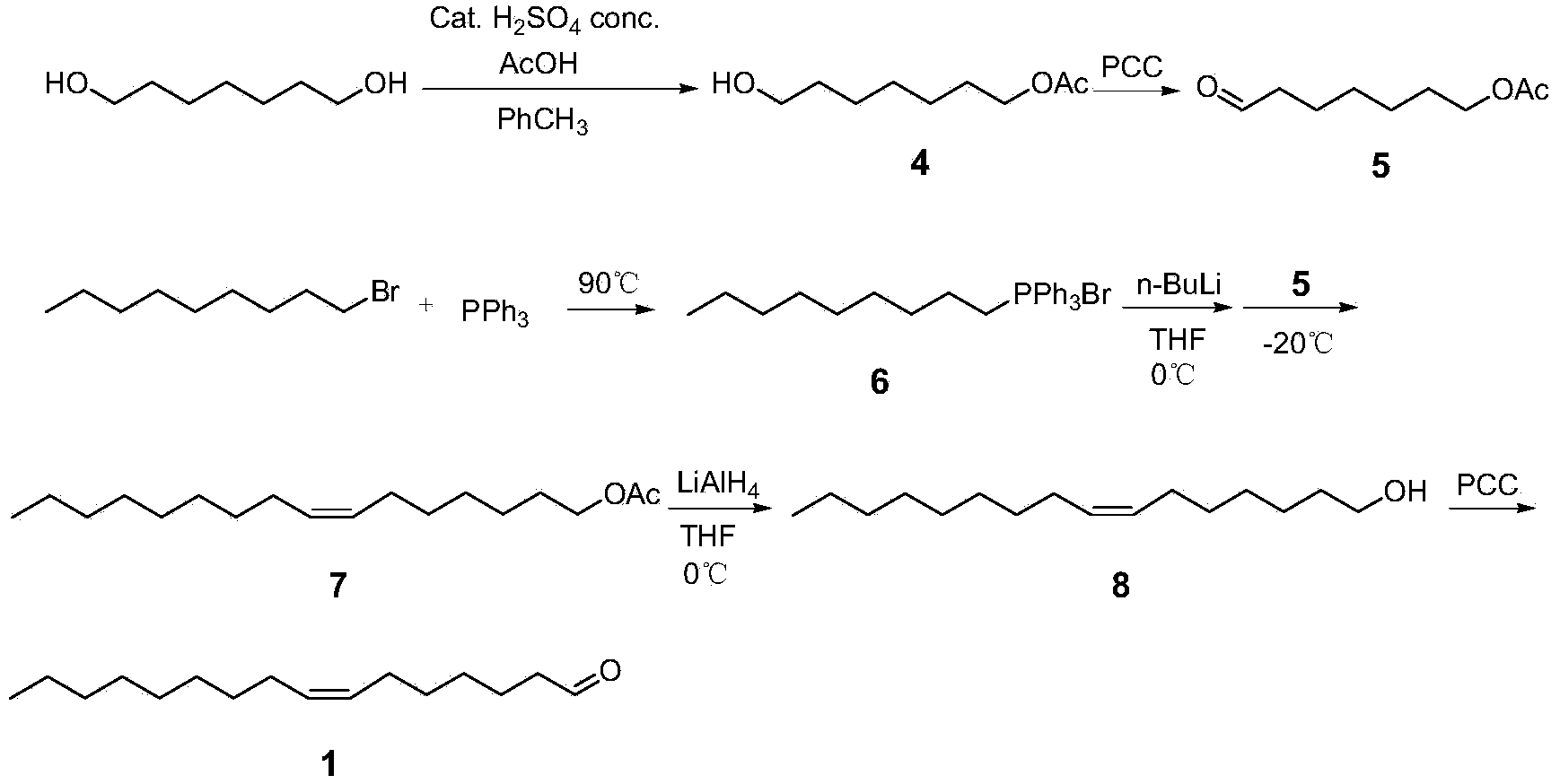
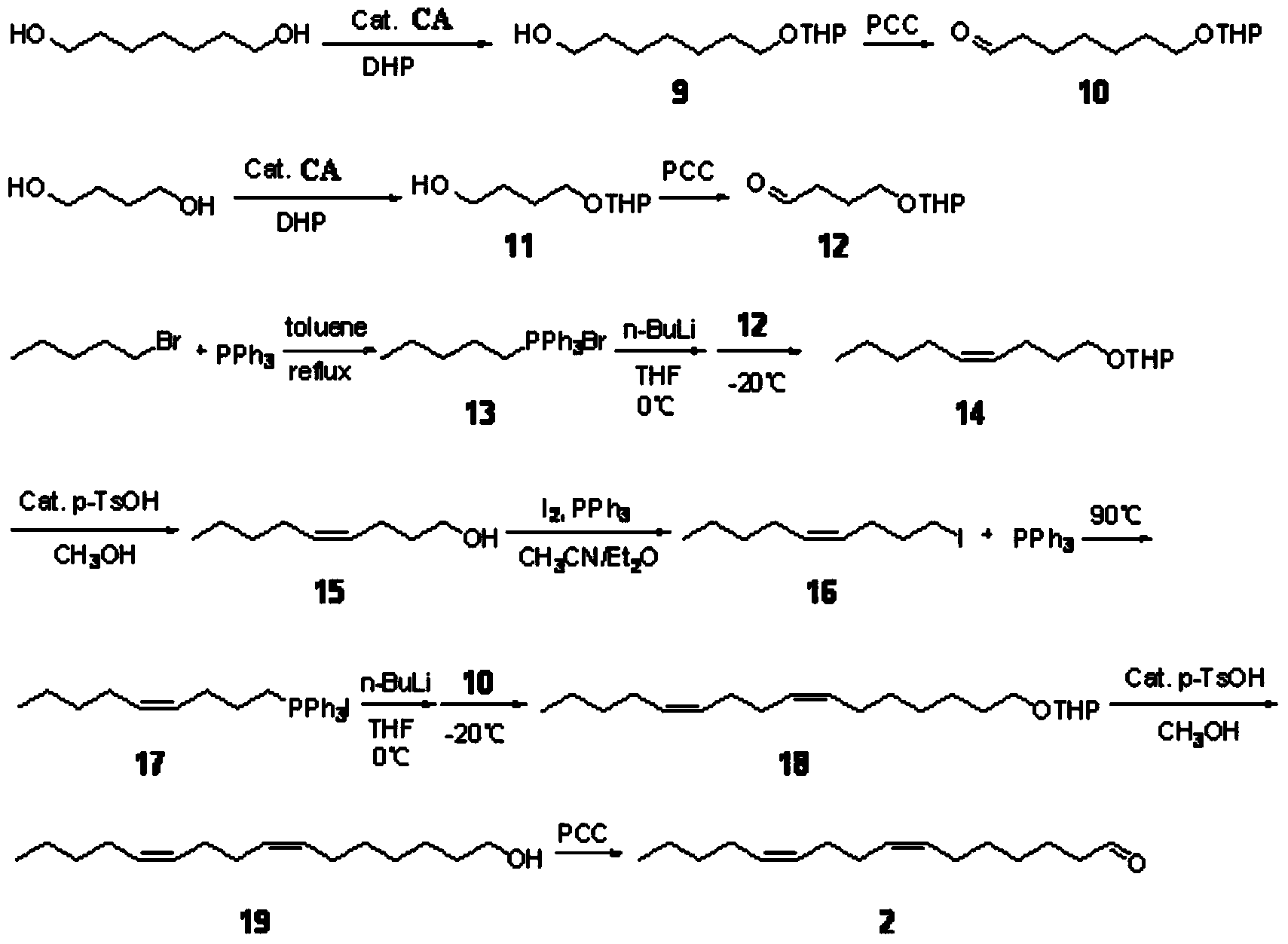
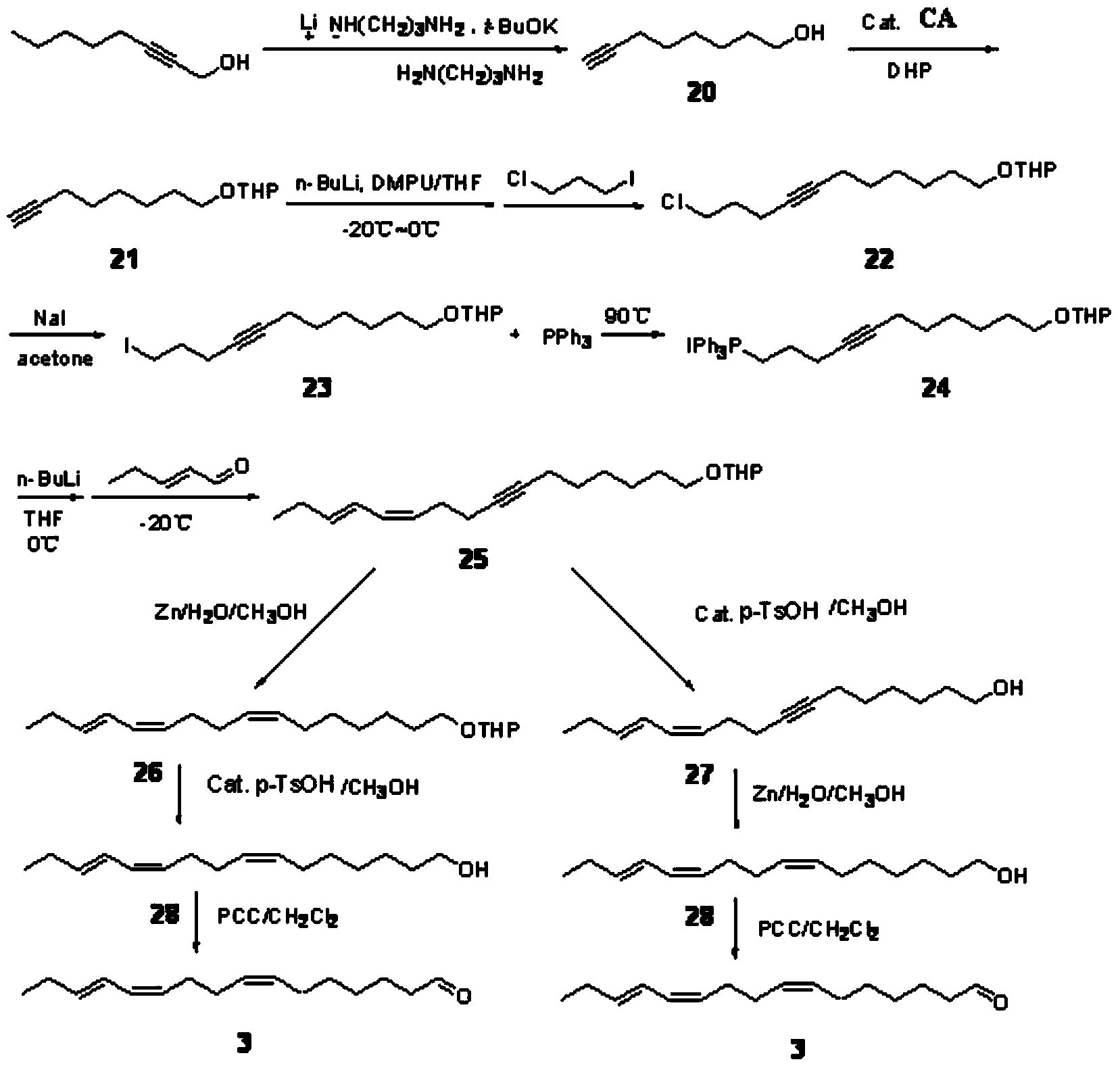
[0045] A method for synthesizing sex pheromone compounds of citrus leafminer, comprising three compounds (Z)-7-hexadecenal, (Z, Z)-7,11-hexadecanal in citrus leafminer sex pheromone The synthetic method of carbadienal and (Z, Z, E)-7,11,13-hexadecatrienal;
[0046] A kind of synthetic method of (Z)-7-hexadecenal, its concrete steps are: take 1,7-heptanediol and brominated n-nonane as starting raw materials; 1,7-heptanediol undergoes unilateral Protection and oxidation reaction to obtain heptanal with unilateral hydroxyl protection; n-nonyltriphenylphosphine bromide obtained from the reaction of brominated n-nonane and triphenylphosphine after alkali treatment, and heptanal with unilateral hydroxyl protection Wittig reaction, the product obtained is through deprotection and oxidation reaction, synthesized (Z)-7-hexadecenal; chemical reaction equation is:
[0047]
[0048] Synthesis of Compound 4: Add 16.2 g (0.12 mol) of diol and toluene (500 mL) into a 1L three-necked flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com