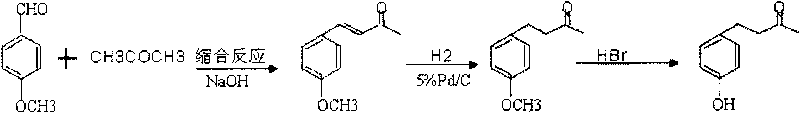New method for synthesizing raspberry ketone by using natural equivalent anisic aldehyde
A technology of raspberry ketone and anisaldehyde, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve the problems of consumption and environmental protection, and achieve low energy consumption and synthetic process route Innovation, the effect of short lines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] 1. Preparation of 4-(4-methoxyphenyl)-3-buten-2-one from natural equivalent anisaldehyde:
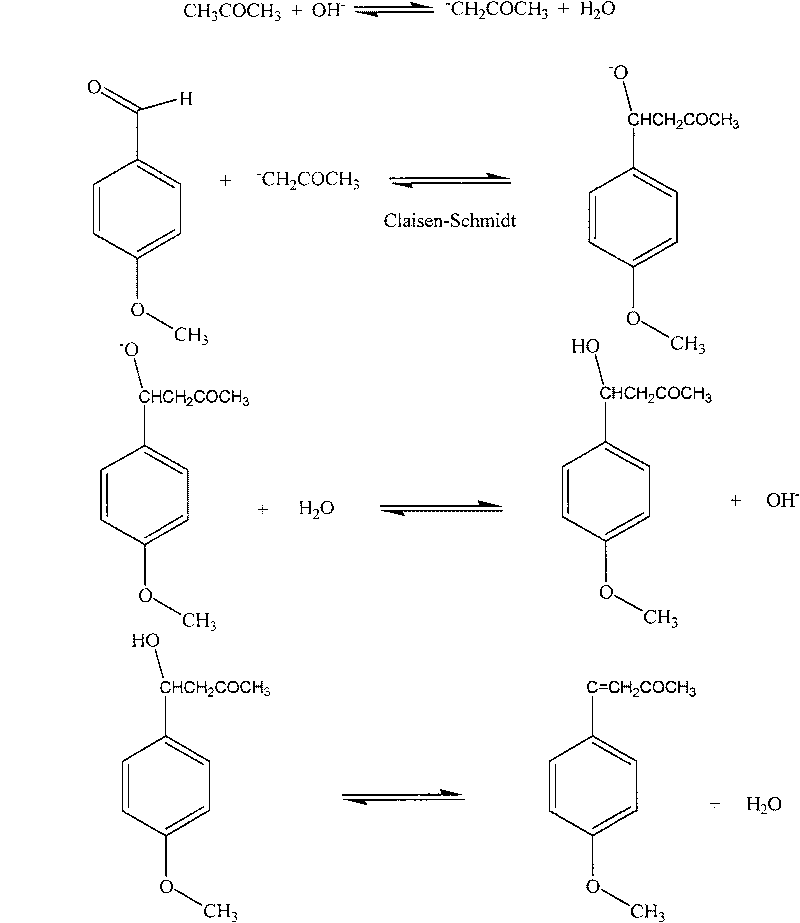
[0017] Natural equivalent anisaldehyde is raw material (28.4g, 0.2086mol), add water (30ml), sodium hydroxide (8.0g), acetone (30ml), reaction temperature is between 20-40 ℃, react for 5.5 hours, separate The oil phase was dried overnight with anhydrous magnesium sulfate; the solvent was removed by rotary evaporation to obtain the (Claisen-Schmidt) condensation product: 4-(4-methoxyphenyl)-3-buten-2-one.
[0018] 2. Preparation of anisyl acetone by catalytic hydrogenation reduction:
[0019] The product 4-(4-p-methoxyphenyl)-3-buten-2-one is dissolved in ethanol, palladium carbon is added for catalysis, hydrogen is passed into the reaction solution and the hydrogen pressure is kept between 0-0.1MPa, React at 20-40°C (room temperature) for 2 hours, then recover ethanol and distill to obtain anisyl acetone. The weight ratio of each component is: 4-(4-methoxyphenyl)-3-buten-2-one:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com