Synthesis method of alkyl phosphinic acid and aluminium salt thereof under normal-pressure mild condition
A technology for the synthesis of alkylphosphinic acid and its synthesis method, which is applied in the field of synthesis of alkylphosphinic acid and its aluminum salt, which can solve the problems of high environmental protection pressure, increased reaction risk coefficient, and limitation, and achieve high safety factor and high reaction efficiency. Low risk, avoid concentrated effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
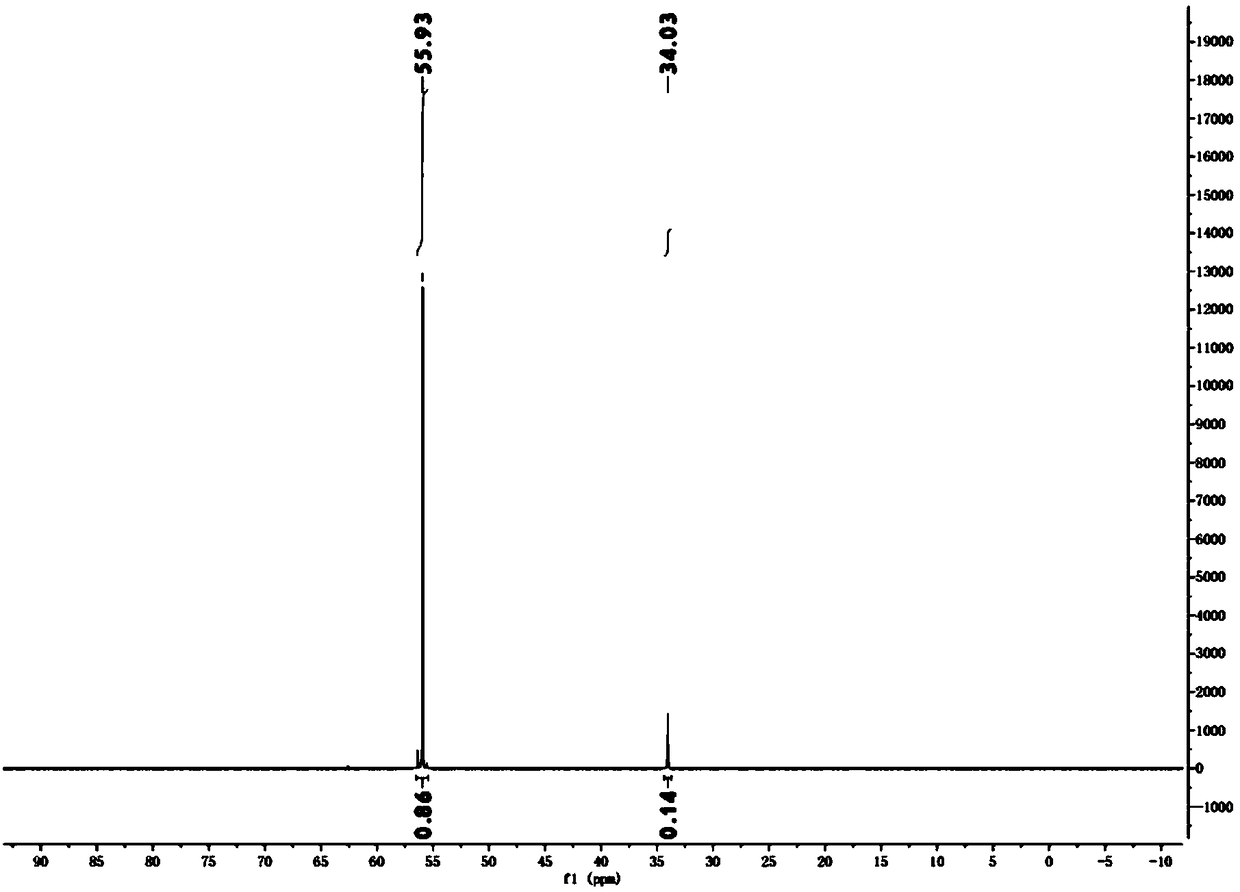
[0028] 10.56 kg of hypophosphorous acid (50% aqueous solution) and 9.0 kg of tert-butanol were added to the reactor. At 70° C., 10.4 kg of chlorobenzene solution containing 0.4 kg of azobisisobutyronitrile and 0.96 kg of concentrated sulfuric acid were added dropwise to the system within 8 hours. Add 6.0 kg of tert-butanol to the system, and add 5.2 kg of chlorobenzene solution containing 0.2 kg of azobisisobutyronitrile and 0.48 kg of concentrated sulfuric acid dropwise within 4 hours at 70°C. At 70°C, the reaction was continued for 12h. After the reaction, the system was spin-dried to obtain a colorless oily substance, which was isobutylphosphinic acid. conduct it 31 PNMR (THF in situ) detection shows that the raw material hypophosphorous acid has been basically completely converted, the content of diisobutyl substituted phosphinic acid is about 86%, and the content of monoisobutyl substituted phosphinic acid is about 14%. (See figure 1 )
[0029] Add 50L of water to th...
Embodiment 2
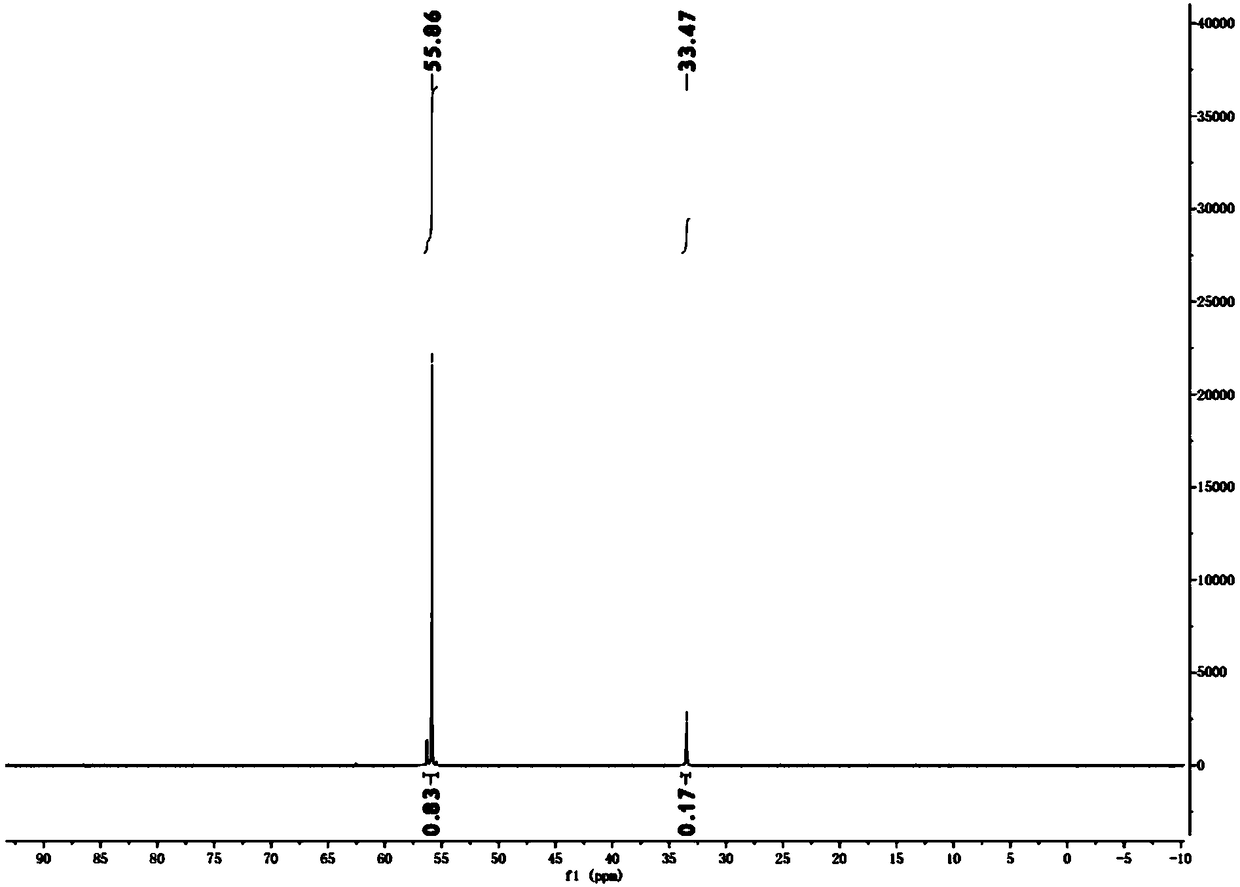
[0031] 10.56 kg of hypophosphorous acid (50% aqueous solution) and 9.0 kg of tert-butanol were added to the reactor. At 70° C., 10.4 kg of recovered chlorobenzene solution containing 0.4 kg of azobisisobutyronitrile and 0.96 kg of concentrated sulfuric acid were added dropwise to the system within 8 hours. Add 6.0 kg of tert-butanol to the system, and add 5.2 kg of recovered chlorobenzene solution containing 0.2 kg of azobisisobutyronitrile and 0.48 kg of concentrated sulfuric acid dropwise within 4 hours at 70°C. At 70°C, the reaction was continued for 12h. After the reaction, the system was spin-dried to obtain a colorless oily substance, which was isobutylphosphinic acid. conduct it 31 PNMR (THF in situ) detection shows that the raw material hypophosphorous acid has been basically completely converted, the content of diisobutyl substituted phosphinic acid is about 83%, and the content of monoisobutyl substituted phosphinic acid is about 17%. (See image 3 )
[0032] Ad...
Embodiment 3
[0034] 10.56 kg of hypophosphorous acid (50% aqueous solution) and 9.0 kg of tert-butanol were added to the reactor. At 80° C., 10.4 kg of chlorobenzene solution containing 0.60 kg of dibenzoyl peroxide and 0.96 kg of concentrated sulfuric acid were added dropwise to the system within 12 hours. Add 6.0 kg of tert-butanol to the system, and add dropwise 5.2 kg of chlorobenzene solution containing 0.30 kg of dibenzoyl peroxide and 0.48 kg of concentrated sulfuric acid at 80° C. within 6 hours. At 90°C, the reaction was continued for 16h. After the reaction, the system was spin-dried to obtain a colorless oily substance, which was isobutylphosphinic acid. conduct it 31 PNMR (THF in situ) detection shows that the raw material hypophosphorous acid has been basically completely converted, the content of diisobutyl substituted phosphinic acid is about 82%, and the content of monoisobutyl substituted phosphinic acid is about 18%.
[0035] Add 50L of water to the oil, and adjust the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com