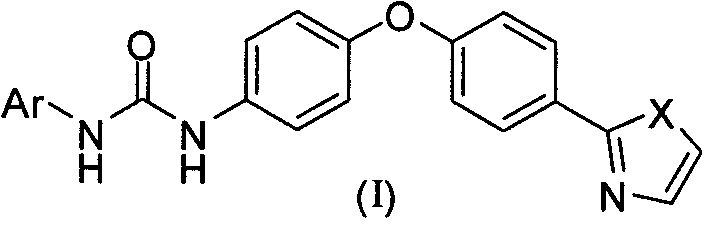
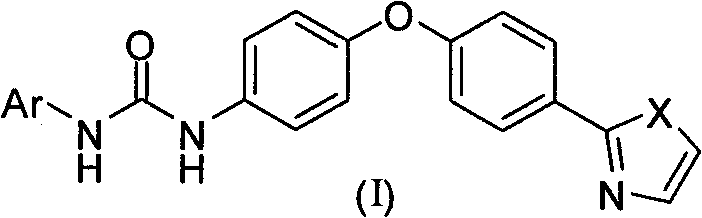
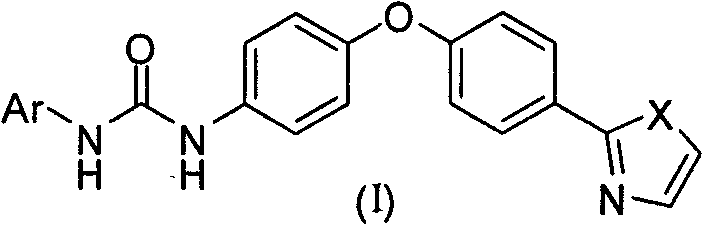
Diaryl urea derivative and application thereof
An aryl and phenyl technology, which is applied in the field of synthesis and preparation of tyrosine kinase inhibitors, can solve the problems of poor treatment effect of AML patients and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] 2-[4-(4-Aminophenoxy)phenyl]thiazole (5)
[0088]
[0089] step 1
[0090] Add 60g (0.75mol) of 70% sodium hydrosulfide, 76g (0.375mol) of magnesium chloride hexahydrate, and 50g (0.375mol) of p-methoxybenzonitrile into a 1L three-necked flask, dissolve in 500mL DMF, and stir mechanically at room temperature for 8h. Stop the reaction, pour the reaction solution into 1L of water, precipitate a yellow solid, filter, suspend the filter cake in 1N dilute hydrochloric acid (1.5L), stir for 30min, filter, and dry to obtain 56.2g of a light yellow solid (1). 89.7%, mp 149-151°C.
[0091] step 2
[0092] 20 g (0.12 mol) of compound (1), 18.6 mL (0.12 mol) of bromoacetaldehyde diethyl acetal, and 500 mL of absolute ethanol were added to a 1L eggplant-shaped bottle, and the mixture was stirred and refluxed for 10 h. The reaction was stopped, the reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, the obtained solid was dissol...
Embodiment 2
[0100] 1-(4-Chlorophenyl)-3-{4-[4-(2-thiazolyl)phenoxy)]phenyl}urea (Ia-1)
[0101]
[0102]Add 0.6g (2.24mmol) of compound (5) into a 100mL three-necked flask, dissolve in 40mL of anhydrous tetrahydrofuran, then add 1.8g (11.2mmol) of N,N'-carbonyldiimidazole (CDI), reflux reaction under nitrogen protection, point After the board monitors that the reaction of the raw materials is complete, 0.36 g (2.35 mmol) of p-chloroaniline is added, and the reflux reaction is continued for 3 h. Stop the reaction, cool the reaction solution to room temperature, evaporate the solvent under reduced pressure, add 50 mL of water to remove excess CDI, extract with ethyl acetate (3×30 mL), combine the organic layers, wash with water (30 mL) and saturated brine (30 mL) respectively , dried over anhydrous sodium sulfate overnight. After filtration, the solvent was distilled off under reduced pressure, and separated by silica gel column chromatography (petroleum ether: ethyl acetate = 2:1 as th...
Embodiment 3
[0104] 1-(4-Chloro-3-trifluoromethylphenyl)-3-{4-[4-(2-thiazolyl)phenoxy)]phenyl}urea (Ia-2)
[0105]
[0106] Using the method of Example 2, input compound (5) 0.6g (2.24mmol) and 4-chloro-3-trifluoromethylaniline 0.46g (2.35mmol), obtain white solid (Ia-2) 0.58g, yield 52.9%, mp 135-136°C. IR (KBr, cm -1 ): 3304.98, 1645.91, 1601.07, 1556.23, 1501.78, 1415.30, 1251.96, 1175.09, 1139.86, 823.38; 1 H-NMR (DMSO-d 6 , 300MHz) δ (ppm): 7.04-7.11 (m, 4H, Ar-H), 7.51-7.54 (m, 2H, Ar-H), 7.59-7.67 (m, 2H, Ar-H), 7.73 (d , J=3.26Hz, 1H, Ar-H), 7.88(d, J=3.25Hz, 1H, Ar-H), 7.92-7.97(m, 2H, Ar-H), 8.11(d, J=2.27Hz , 1H, Ar-H), 8.87(s, 1H,- NH CONH-), 9.14(s, 1H, -NHCO NH -); 13 C-NMR (DMSO-d 6 , 125MHz) δ(ppm): 117.61, 118.17, 119.77, 120.32, 121.75, 127.67, 127.96, 128.69, 136.27, 139.63, 143.60, 149.68, 152.53, 159.40, 166.54; ESI-MS m / z: ] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com