Method for preparing wheat protein source opioid peptide by enzymolysis of wheat protein
A wheat protein and active peptide technology, applied in the direction of fermentation, can solve the problem of less bioactive peptides from wheat gluten protein, and achieve the effect of promoting deep utilization and development, expanding the scope of application, and high opioid activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: prepare wheat protein solution, adopt guinea pig ileum method to measure whether wheat protein has opioid activity, the result shows, even if add test tank and make wheat protein concentration reach 50mg / mL, still do not observe the phenomenon of inhibiting guinea pig ileum contraction, and and There was no significant difference in the blank control group (Tyrode's solution). Wheat protein therefore does not have opioid activity.
Embodiment 2
[0021] Embodiment 2: Use isolated guinea pig ileum to detect the opioid activity of morphine, its IC 50 The value is 0.05 mg / mL.
Embodiment 3
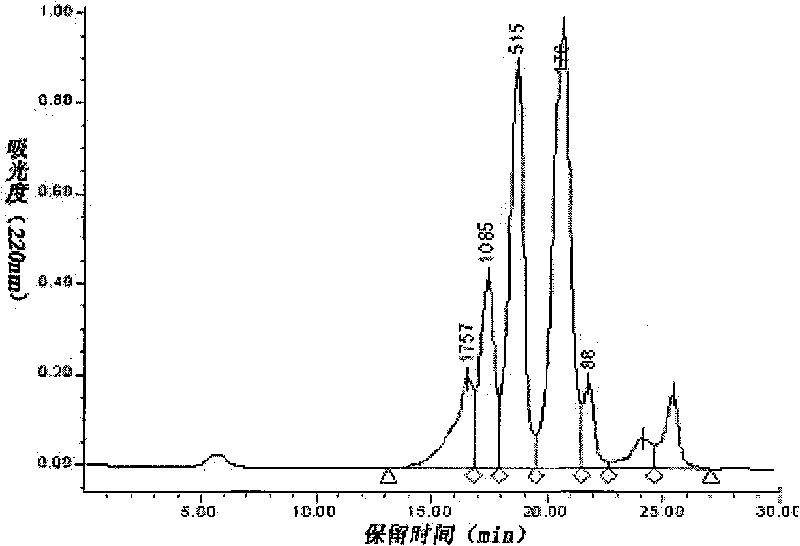
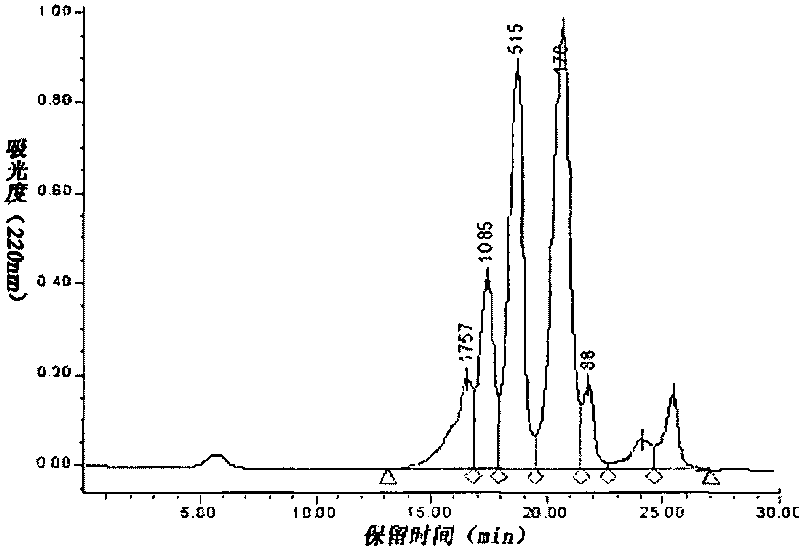
[0022] Example 3: 5.0 g of gluten powder was dispersed in 100 mL of aqueous solution, adjusted to pH 7.0, and stirred for 20 min. Adjust the temperature to 50°C, add neutral protease for enzymolysis, the amount of enzyme added is 1.0% of the weight of gluten, and the hydrolysis time is 12h. After the hydrolysis is completed, the enzyme is inactivated at 95°C for 10 minutes, cooled, centrifuged at 3000rpm for 20 minutes, the supernatant is decolorized by activated carbon adsorption, and separated by a 3000Da ultrafiltration membrane to prepare the wheat peptide product. Using guinea pig ileum method, it was found that the wheat peptide prepared under this condition had no opioid activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com