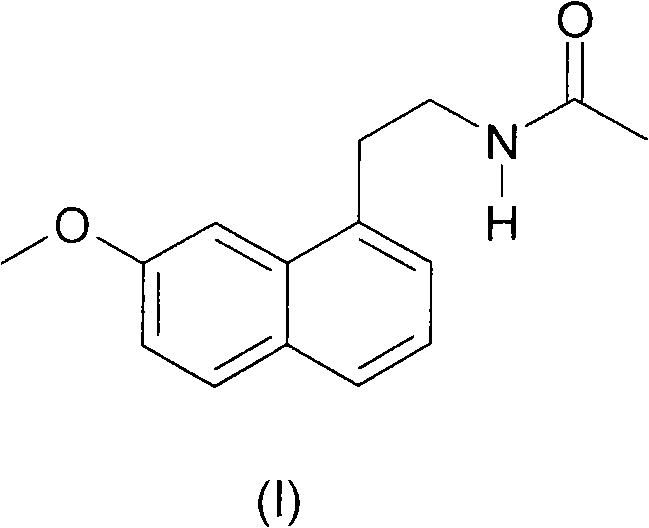
Agomelatine crystal form B, preparation method thereof and medicinal composition containing same
A technology for agomelatine crystals and crystal forms, which is applied in the field of antidepressant drugs, can solve the problems of agomelatine product loss, cumbersomeness, poor reproducibility, etc., and achieves simple and easy operation, difficulty and cost of the method. The effect of reducing and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of agomelatine crystal form B
[0030] Add 100 g of agomelatine and 300 ml of ethanol to the reaction flask, heat and stir to dissolve. Cool the obtained agomelatine ethanol solution to 25±5°C, slowly add 2400ml of drinking water dropwise; after the dropwise addition, filter the precipitated crystals, and vacuum dry to obtain agomelatine 98.7g, yield: 98.7% .
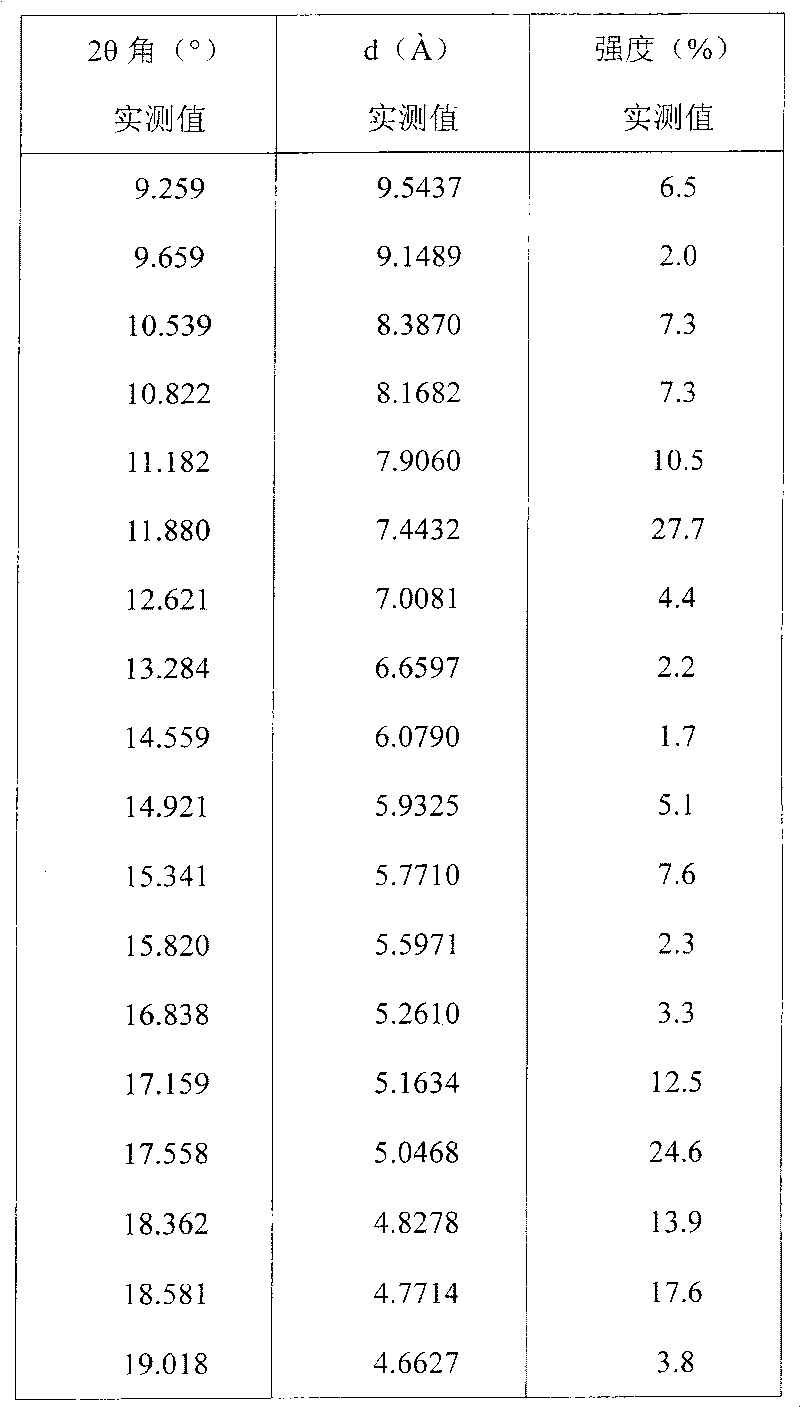
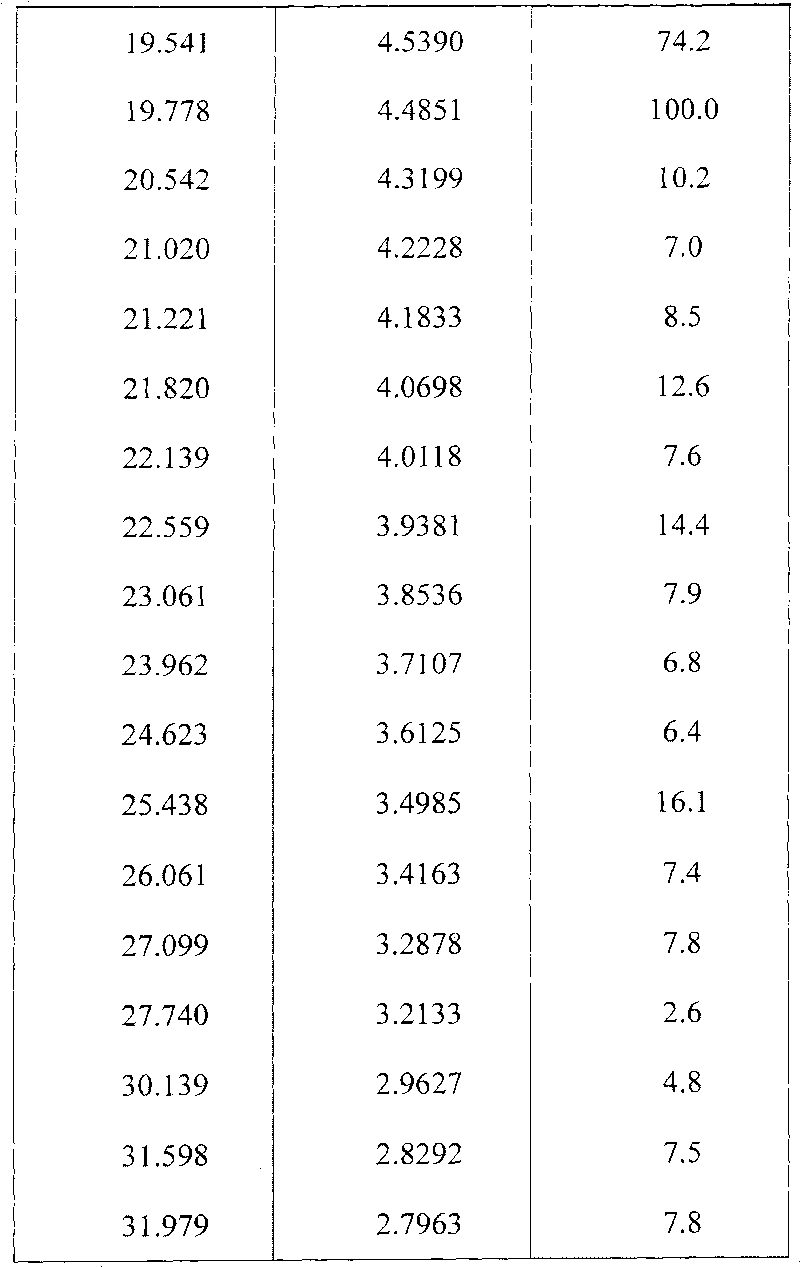
[0031] The resulting product is measured by D / max-rA target X-ray diffractometer (copper to cathode) and expressed as follows with interplanar spacing d, Bragg 2θ angle, intensity and relative intensity (expressed as a percentage of the strongest ray):
[0032]
[0033]
[0034] Embodiment 2: Preparation of agomelatine crystal form B
[0035] Add 100 g of agomelatine and 500 ml of ethanol to the reaction flask, heat and stir to dissolve. Cool the obtained agomelatine ethanol solution to 25±5°C, slowly add 5000ml of drinking water dropwise; after the dropwise addition, filter the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com