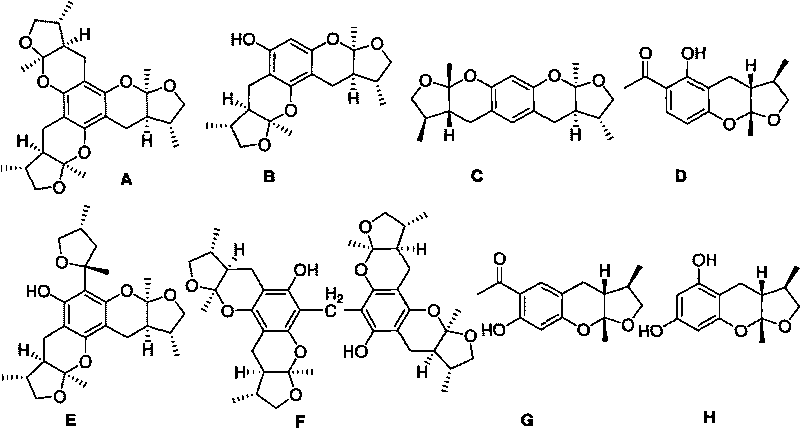
Synthetic method of Xyloketals compounds
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of high production cost and complex process, and achieve the effects of low production cost, simple process and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
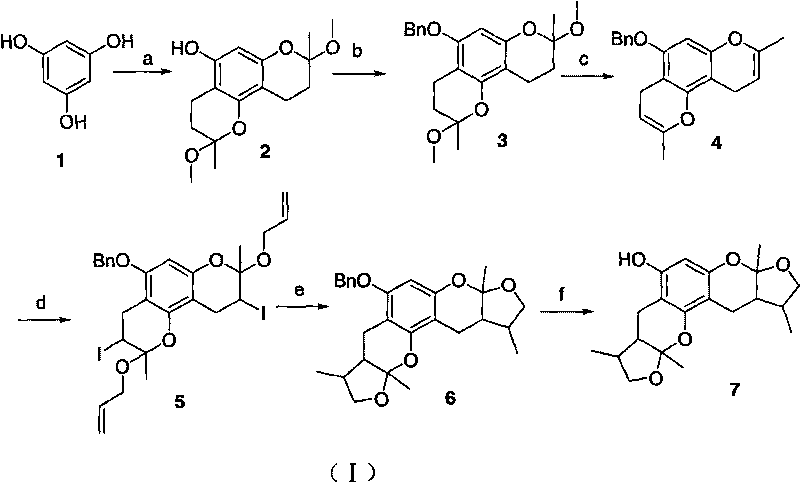
[0022] Example 1: Synthesis of tri-substituted benzopyran cyclic ether
[0023] Add 1.0g (7.94mmol) of phloroglucinol, 2.2g (31.75mmol) of butenone, 2.0g of anhydrous magnesium sulfate to a 50ml round bottom flask, then add 30ml of anhydrous methanol, stir at 0℃ to dissolve, Then slowly add 2.56g (15.88mmol) of p-toluenesulfonic acid, continue to stir the reaction, let it slowly rise to room temperature, and react for 8h. After the reaction is tracked by TLC, 10ml of saturated NH4Cl is added at 0℃ to stop the reaction. 100ml EtOAc, saturated NH 4 Wash with Cl (1×10ml), saturated brine (2×10ml), anhydrous MgSO 4 Drying, concentration under reduced pressure. Column chromatography to obtain 2.85g of white solid, yield 95%. mp 134-135℃; 1 H NMR(CDCl 3 , 400MHz) δ: 3.255 (s, 3H), 3.242 (s, 3H), 3.237 (s, 3H), 2.641 (m, 6H), 2.058 (m, 3H), 1.746 (m, 3H), 1.524 (s , 6H), 1.518 (s, 3H);
Embodiment 2
[0025] 1. Synthesis of disubstituted benzopyran cyclic ether (2)
[0026] Add 1.0g (7.94mmol) of phloroglucinol, 1.3g (18.26mmol) of butenone, 1.5g of anhydrous magnesium sulfate to a 50ml round bottom flask, then add 30ml of anhydrous methanol, stir at 0℃ to dissolve, Then slowly add 1.43 g (7.94 mmol) of p-toluenesulfonic acid, continue to stir the reaction, let it slowly rise to room temperature, and react for 8 hours. After the reaction is tracked by TLC, saturated NH is added at 0°C. 4 Cl 10ml, stop the reaction, add 100ml EtOAc, saturated NH 4 Wash with Cl (1×10ml), saturated brine (2×10ml), anhydrous MgSO 4 Drying, concentration under reduced pressure, column chromatography to obtain 1.68 g of white solid, yield 72%. mp 170-171℃, 1 H NMR(CDCl 3 , 400MHz) δ: 5.997 (s, 1H), 5.167 (s, 1H), 3.270 (s, 3H), 3.260 (s, 3H), 2.649 (m, 2H), 2.604 (m, 2H), 2.101 (m , 1H), 2.069 (m, 1H), 1.792 (m, 1H), 1.730 (m, 1H), 1.542 (s, 3H), 1.518 (s, 3H);
[0027] 2. Protection of disubstituted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com