Multi-functional group resin catalyst and preparation method thereof
A multi-functional, catalytic technology, applied in the direction of catalyst activation/preparation, hydroxyl addition preparation, chemical instruments and methods, etc., can solve the problems of catalytic activity impact, poor affinity, etc., and achieve low shedding rate, expanded production capacity, and low energy consumption Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
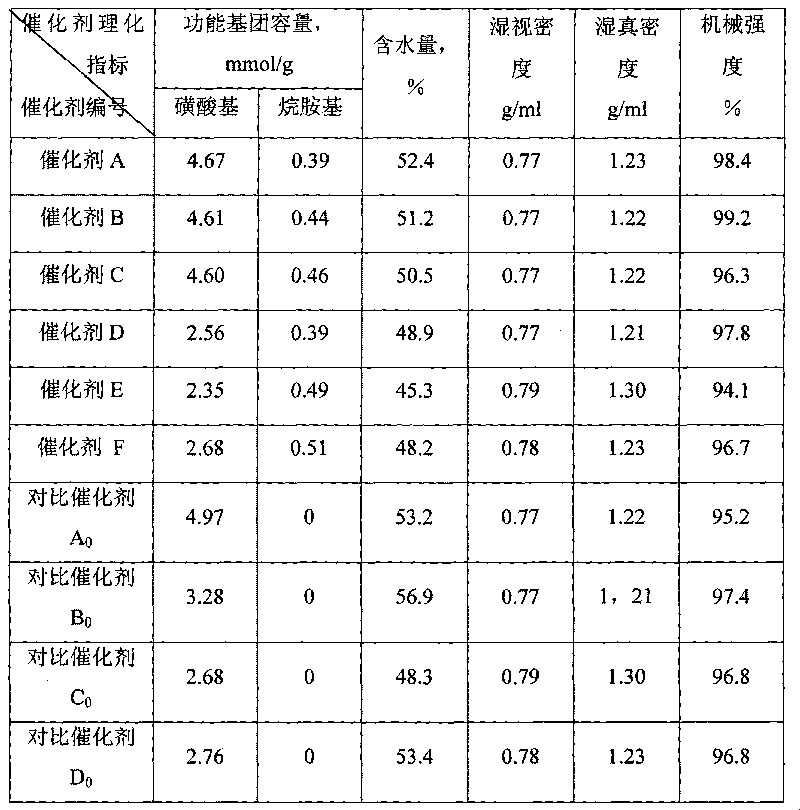
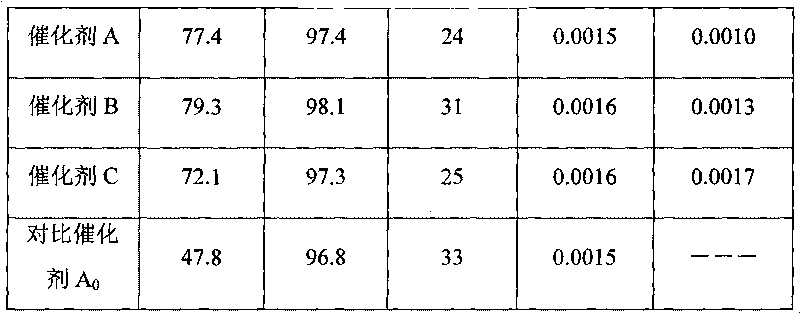
[0023] (1) Chlorosulfonation of polystyrene microspheres: Take 100 g of styrene / divinylbenzene copolymer microspheres (particle size 0.40-0.60 mm) with a cross-linking degree of 16% and add them to a 2000 ml reactor, and add 500 ml of chlorosulfonate respectively acid and 40ml dichloroethane, stirred and swelled for 2 hours, heated to 50°C and kept for 20 hours to obtain 146.5g sulfonyl chloride SO 2 Sulfonylated polystyrene microspheres with a Cl content of 45.6% (product 1).
[0024] (2) N-acylation reaction of product 1: 146.5g of product 1 was added to a 2000ml reaction kettle, 450ml of dichloroethane and 7.6g of dodecylamine were added respectively, the temperature was raised to 65°C, and the temperature was kept for 17 hours to obtain 153.3 g of N-acylated product 2.
[0025] (3) Hydrolysis reaction of product 2: Add 153.3g of product 2 into a 2000ml reaction kettle, add 400ml of pure water, stir and heat up to 95°C, and keep it warm for 10 hours to obtain 375.6g of the...
Embodiment 2
[0027] The same process as in Example 1, except that the styrene / divinylbenzene copolymer microspheres are styrene / dipropylenebenzene copolymers with a crosslinking degree of 8%, and 7.6g dodecane will be added in the N-acylation reaction Base amine was changed to add N, N-dihexadecylamine 30g to obtain 389.4g final product---catalyst B, whose physical and chemical indexes recorded are listed in Table 1.
Embodiment 3
[0029] The same process as in Example 1, except that the styrene / divinylbenzene copolymer microspheres are naphthalene ethylene / divinylbenzene copolymers with a degree of crosslinking of 20%, and 8.9 g of nonylamine and The mixture of 23g tetradecylamine, obtains 411.6g final product---catalyst C, and its recorded physicochemical index is listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com