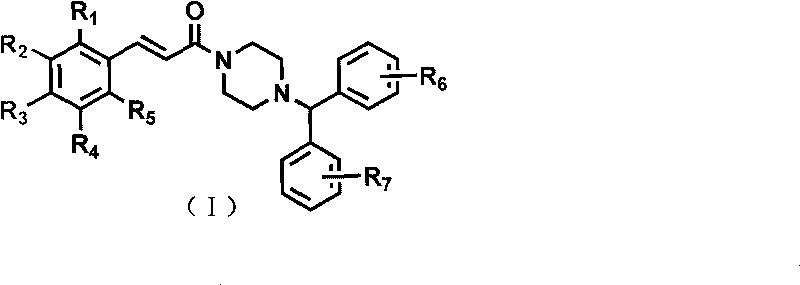
Cinnamamide derivative and application as cerebral nerve protective agent
A technology of cinnamamide and derivatives, applied in the field of substituted cinnamamide derivatives and brain neuroprotectants, can solve the problems of low selectivity, poor oral bioavailability, high neurobehavioral toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (E)-3-(3,4-diacetoxyphenyl)acrylic acid
[0043]Add 3,4-dihydroxybenzaldehyde (13.8g, 0.1mol), malonic acid (15.6g, 0.15mol), pyridine (68ml), hexahydropyridine ( 1.8ml), heated to 95°C for 3 hours. After cooling, the solvent was evaporated under reduced pressure, and the residue was poured into a mixture of concentrated hydrochloric acid (68ml) and ice (150ml) and stirred to precipitate an off-white solid. Filtration and recrystallization from absolute ethanol yielded 6.4 g of off-white powder caffeic acid, yield 35.2%, mp 205.9-209.5°C.
[0044] Add caffeic acid (5.2g, 0.029mol), acetic anhydride (24ml), and pyridine (20ml) into the reaction flask, and stir overnight at room temperature. The solvent was evaporated under reduced pressure, and the residue was poured into ice water to obtain a pale yellow solid, which was recrystallized from absolute ethanol to obtain 6.5 g of a white solid, yield 85.9%, mp 197.1-199.7°C.
Embodiment 2
[0046] (E)-3-(3-methoxy-4-acetoxyphenyl)acrylic acid
[0047] The preparation method is the same as in Example 1. Vanillin (15.2g, 0.1mol), malonic acid (15.6g, 0.15mol), pyridine (68ml), and hexahydropyridine (1.8ml) are heated to 95°C for reaction to obtain A Ferulic acid 9.2g, yield 47.4%, mp170.7-172.4°C.
[0048] Ferulic acid (5.6g, 0.029mol), acetic anhydride (12ml), and pyridine (20ml) were reacted at room temperature to obtain 6.1g of white solid, yield 89.1%, mp 168.5-169.9°C.
Embodiment 3
[0050] (E)-3-(4-fluorophenyl)acrylic acid
[0051] The preparation method is the same as in Example 1, 4-fluorobenzaldehyde (12.4g, 0.1mol), malonic acid (15.6g, 0.15mol), pyridine (68ml), hexahydropyridine (1.8ml), heated to 95°C for reaction, 13 g of white solid was obtained, yield 78.3%, mp 209.1-210.8°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com