Preparation method of rhinal ion sensitive in situ gel
An ion-sensitive, gel-based technology that is applied to non-active ingredient medical preparations, active ingredient-containing medical preparations, and pharmaceutical formulations to improve transolfactory mucosal transport, reduce escape latency, and reduce brain tissue water content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 The preparation method of ion-sensitive in-situ gel of the present invention
[0045] prescription:
[0046] Gardenia iridoid extract: 40g
[0047] Gellan gum: 2g
[0048] Glucose: 30g
[0049] Methylparaben: Appropriate amount
[0050] Trishydroxymethylaminomethane: appropriate amount
[0051] Purified water 1000ml
[0052] The above raw materials and auxiliary materials are made into 1000ml altogether.
[0053] Preparation:
[0054] Take the above raw materials and auxiliary materials, add 400ml of water to the gardenia iridoid extract to dissolve; add 600ml of water to gellan gum, osmotic pressure regulator and preservative, and heat to dissolve at 90°C; mix the two solutions evenly under stirring , adjust the pH value to 7.0±0.2, filter and fill to obtain the spray.
[0055] Shake well before use to form a solution, and press the valve of the nozzle to spray out a considerable amount of drug particles. When in use, point the special nasal spray...
Embodiment 2
[0058] Embodiment 2 The research of ion-sensitive in-situ gel preparation of the present invention
[0059] 1. Dosage Form Study of Nasal Preparations
[0060] Requirements for an ideal preparation for the treatment of brain diseases by nasal administration (brain targeting): ① can reach the olfactory area of the nasal cavity smoothly; ② can be transported across the olfactory mucosa (increase drug absorption); mucosal residence time) ④ low toxicity to cilia.
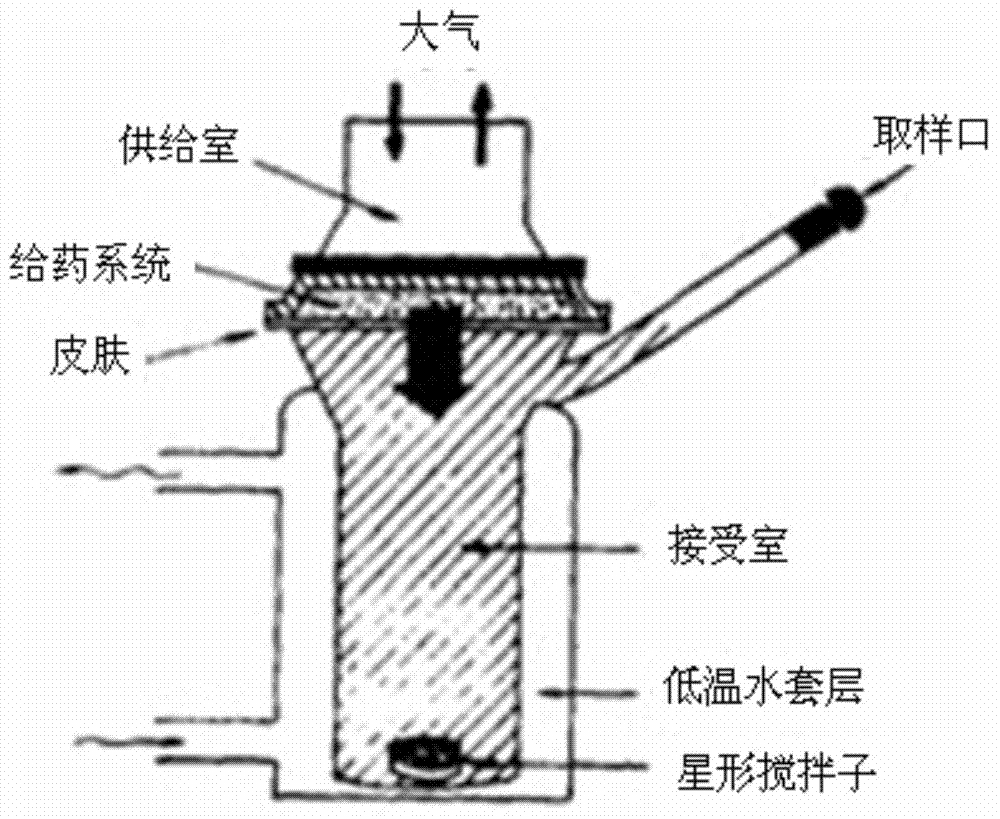
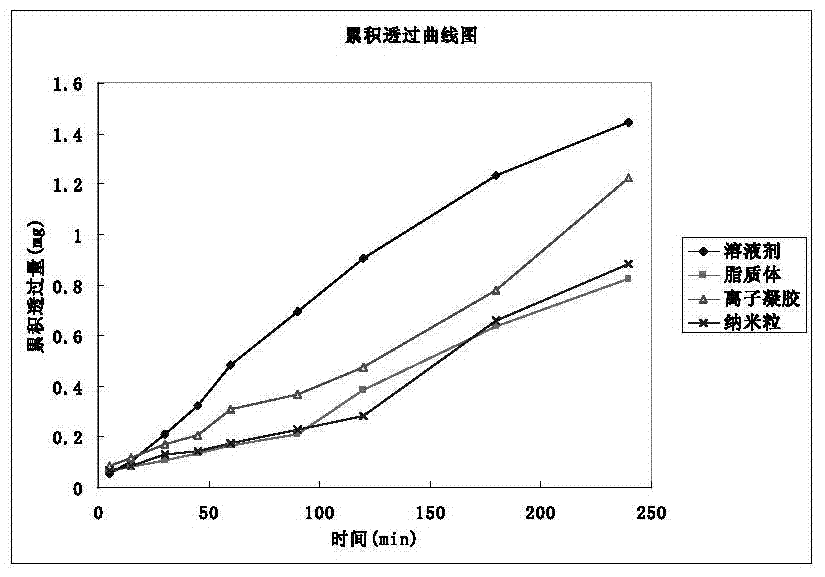
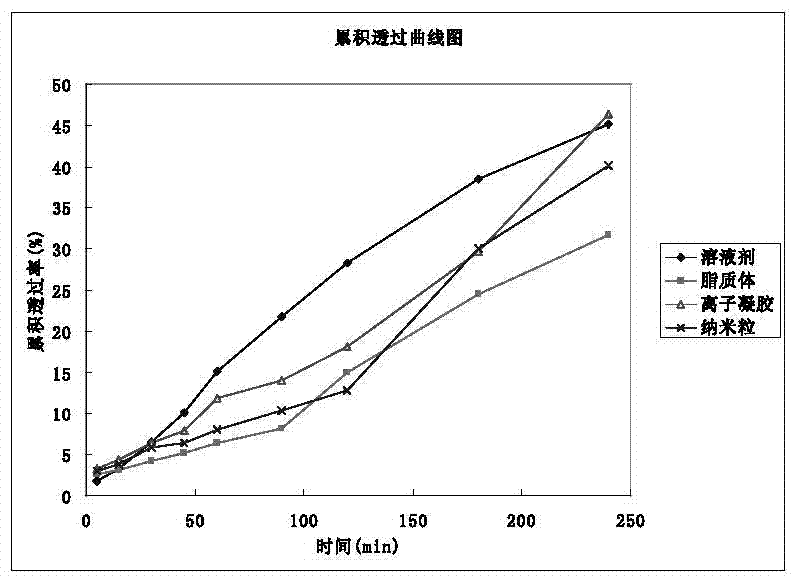
[0061] According to the above requirements, the nature of the drug (water solubility) and the feasibility of mass production at present, it is planned to be developed into a nasal spray. Four dosage forms of nasal solution, gel, nasal liposome and nasal nanoparticles were compared and studied. The dosage forms were screened with the results of cumulative permeabilized porcine olfactory mucosa diffusion, cilia toxicity test and hemolysis test as evaluation indexes.
[0062] 1 Preparation of four preparations
[006...
Embodiment 3
[0176] Example 3 Detection of ion-sensitive in-situ gels of the present invention
[0177] 1. pH measurement
[0178] Randomly select 10 bottles of the preparation and measure the pH value. The results are shown in Table 14 below.
[0179] Table 14 pH value measurement result table
[0180]
[0181] Since nasal administration preparations act directly on the surface of the nasal mucosa, pH value and mucosal irritation must be considered. The pH environment of the human nasal cavity is between 4.5 and 6.5. In order to avoid stimulating the nasal mucosa, the pH value of nasal preparations is generally between 5 and 9. According to the above measurement, the pH of the gel prepared by the present invention is 7.0±0.2.
[0182] 2. Injection rate test:
[0183] According to the non-quantitative spray inspection method under the item Z spray in Appendix I of the Chinese Pharmacopoeia in 2010, 4 bottles of this product were randomly selected, the caps were removed, and they we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com