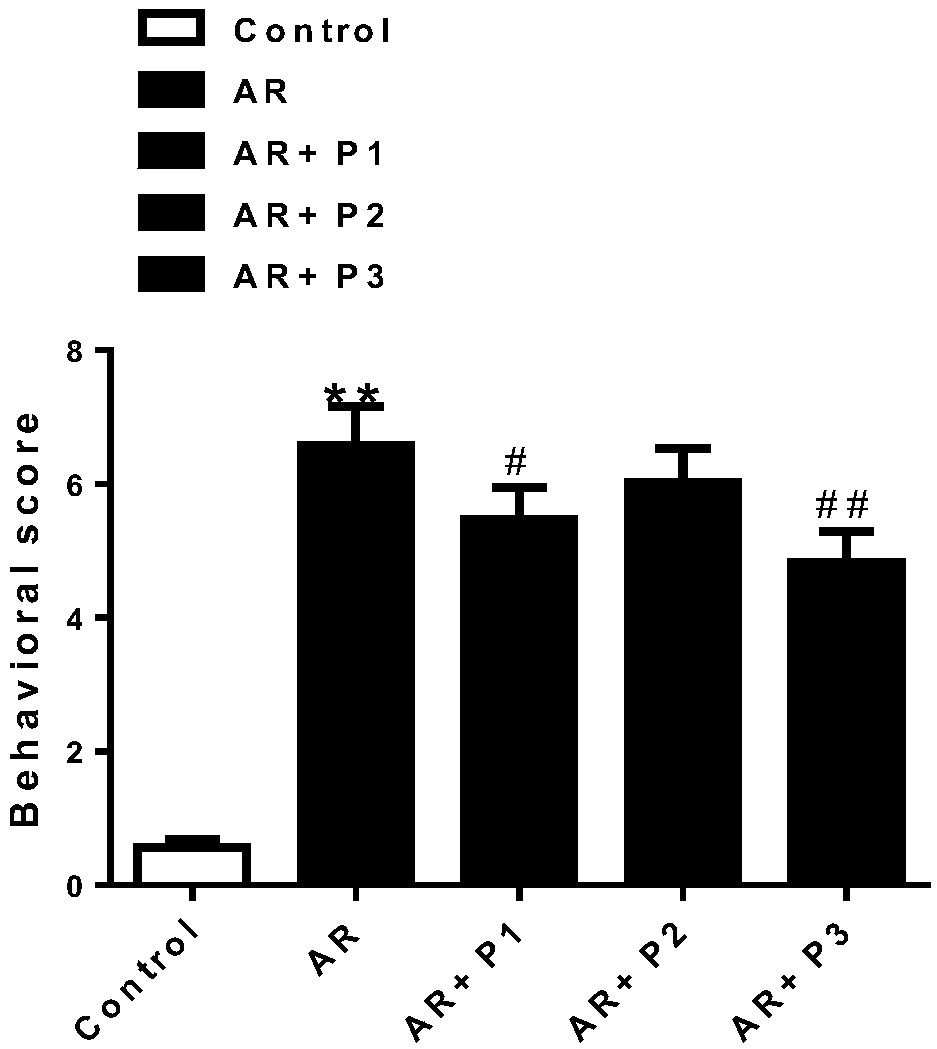
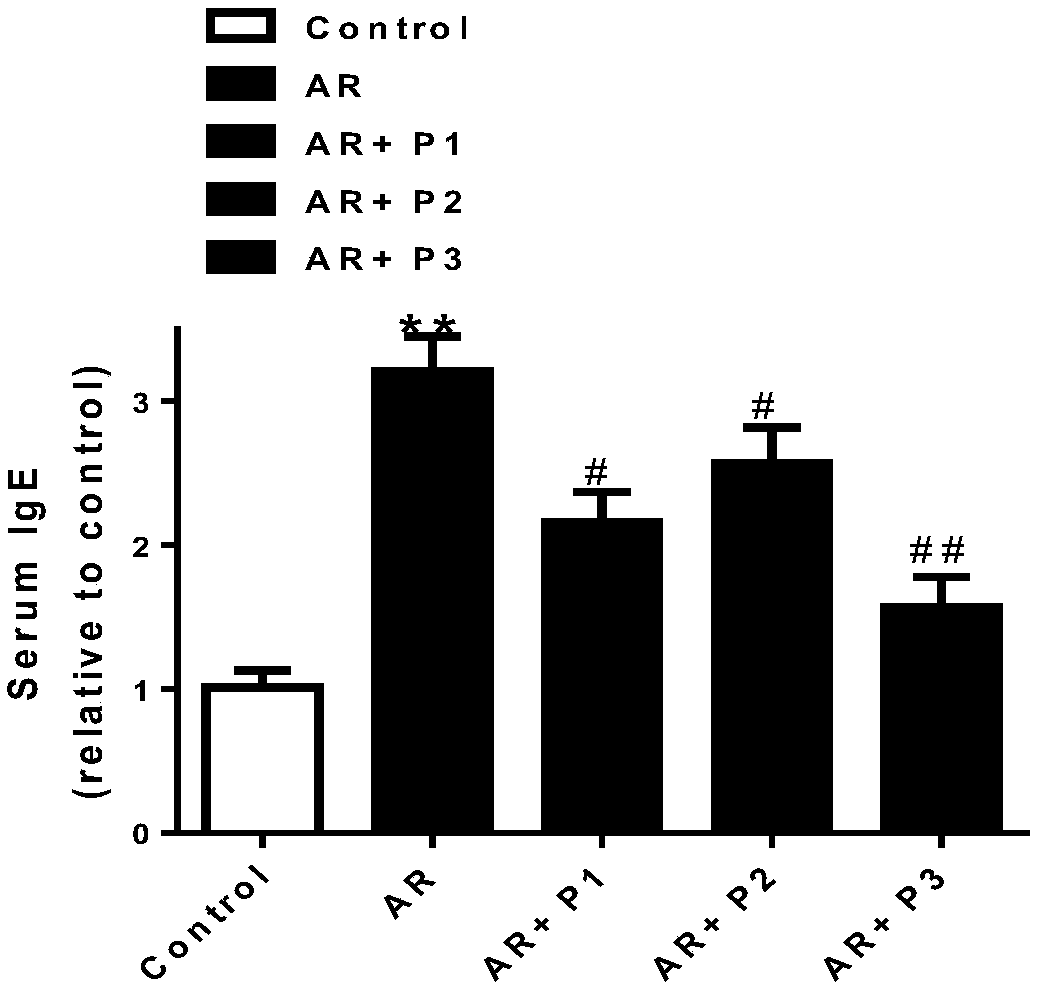
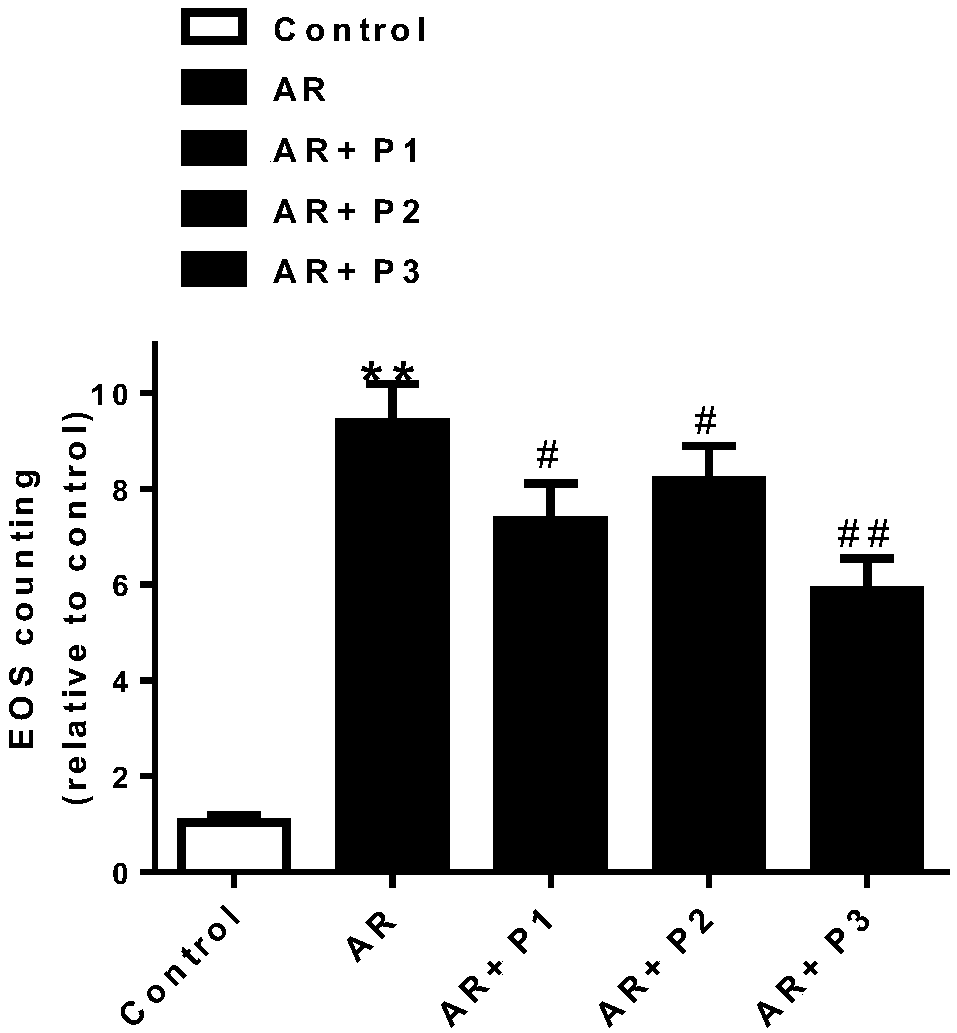
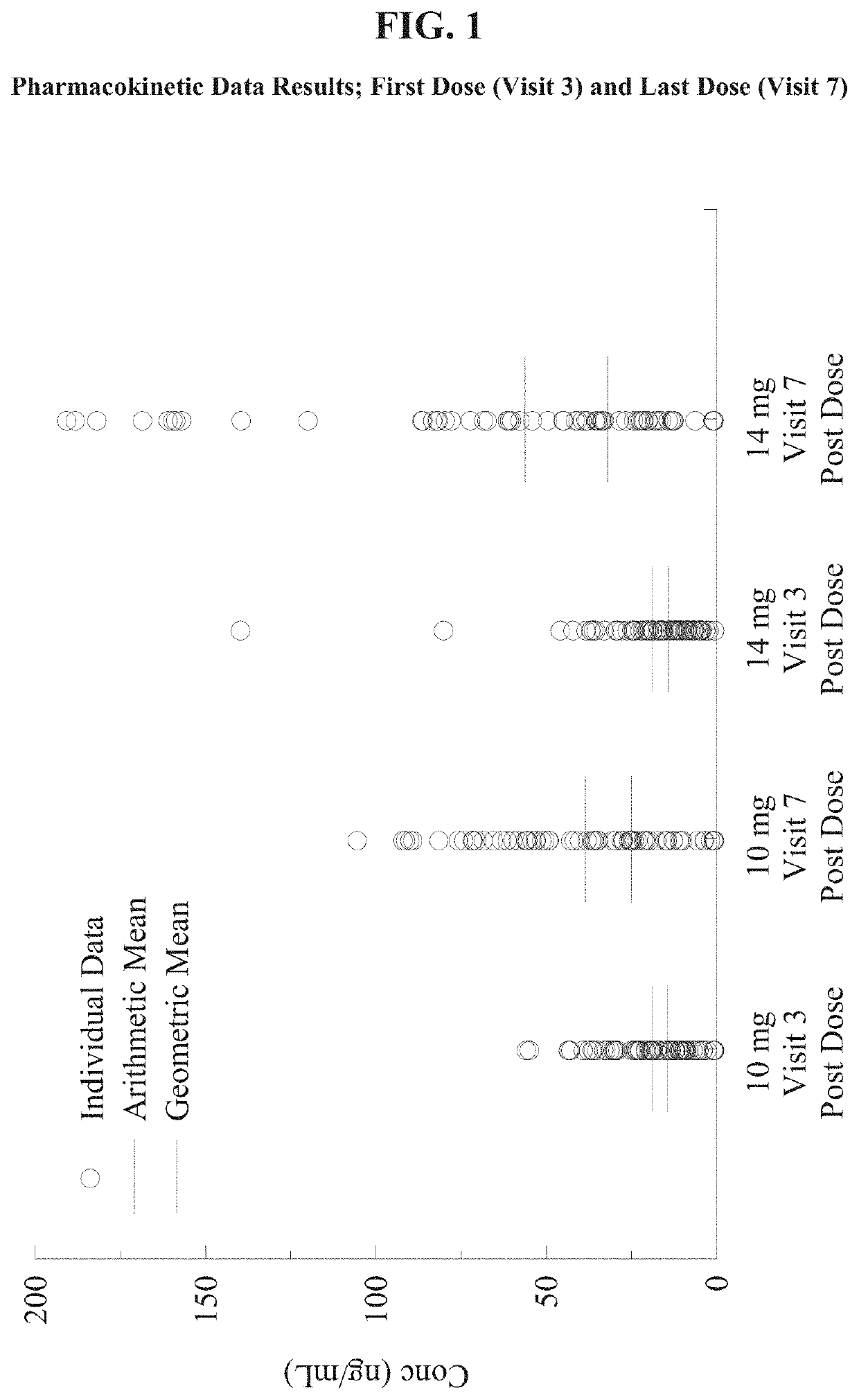
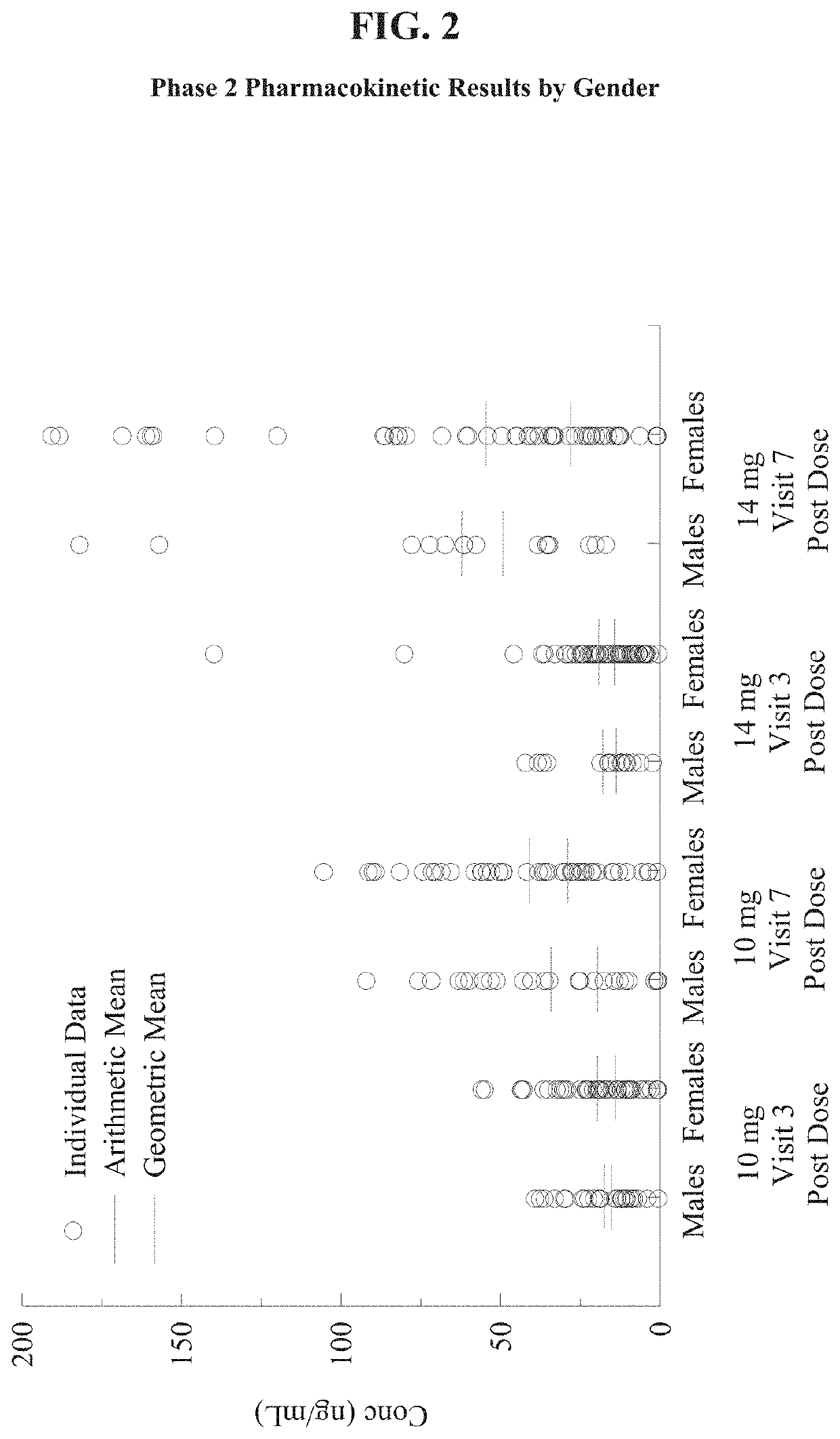
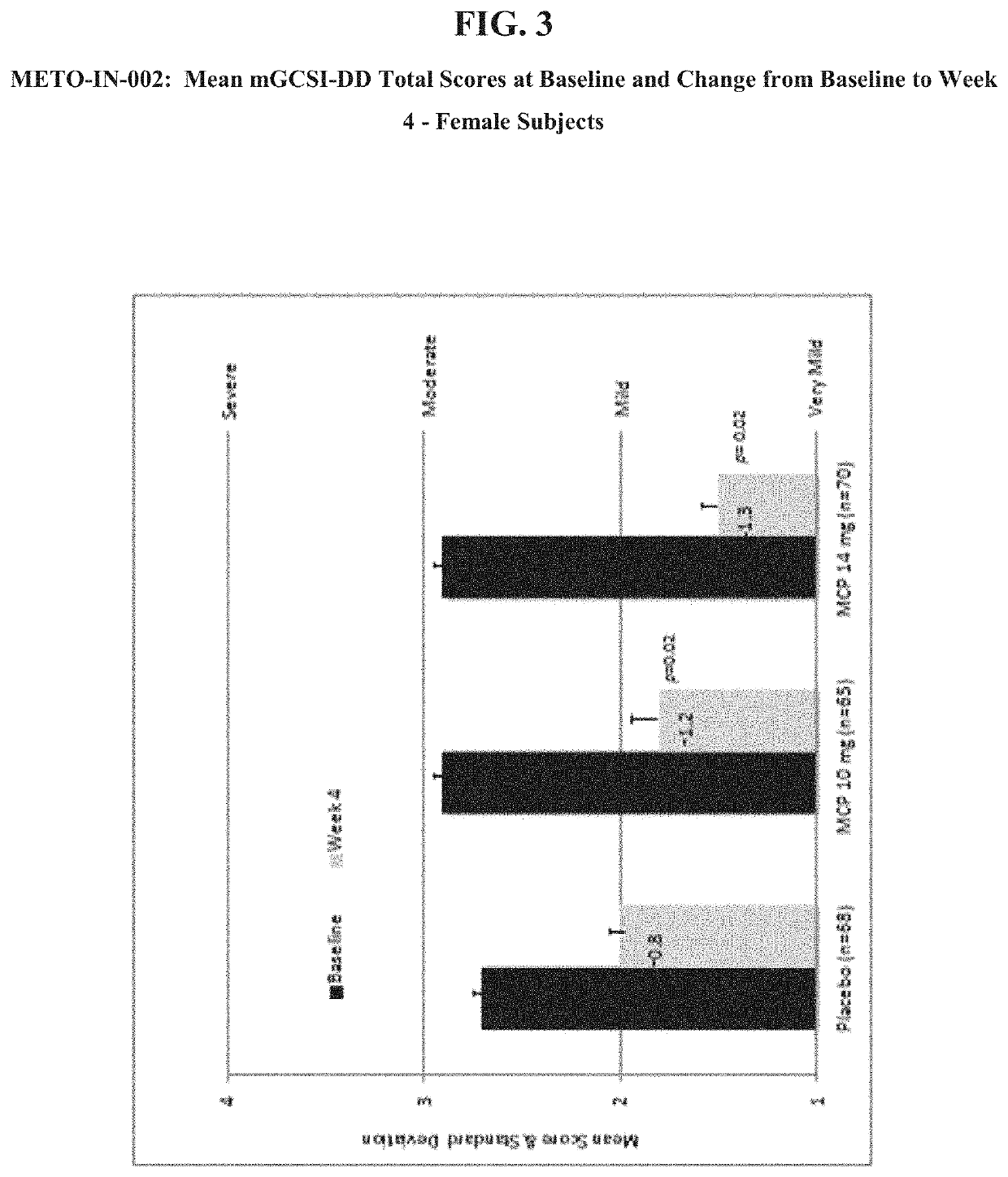
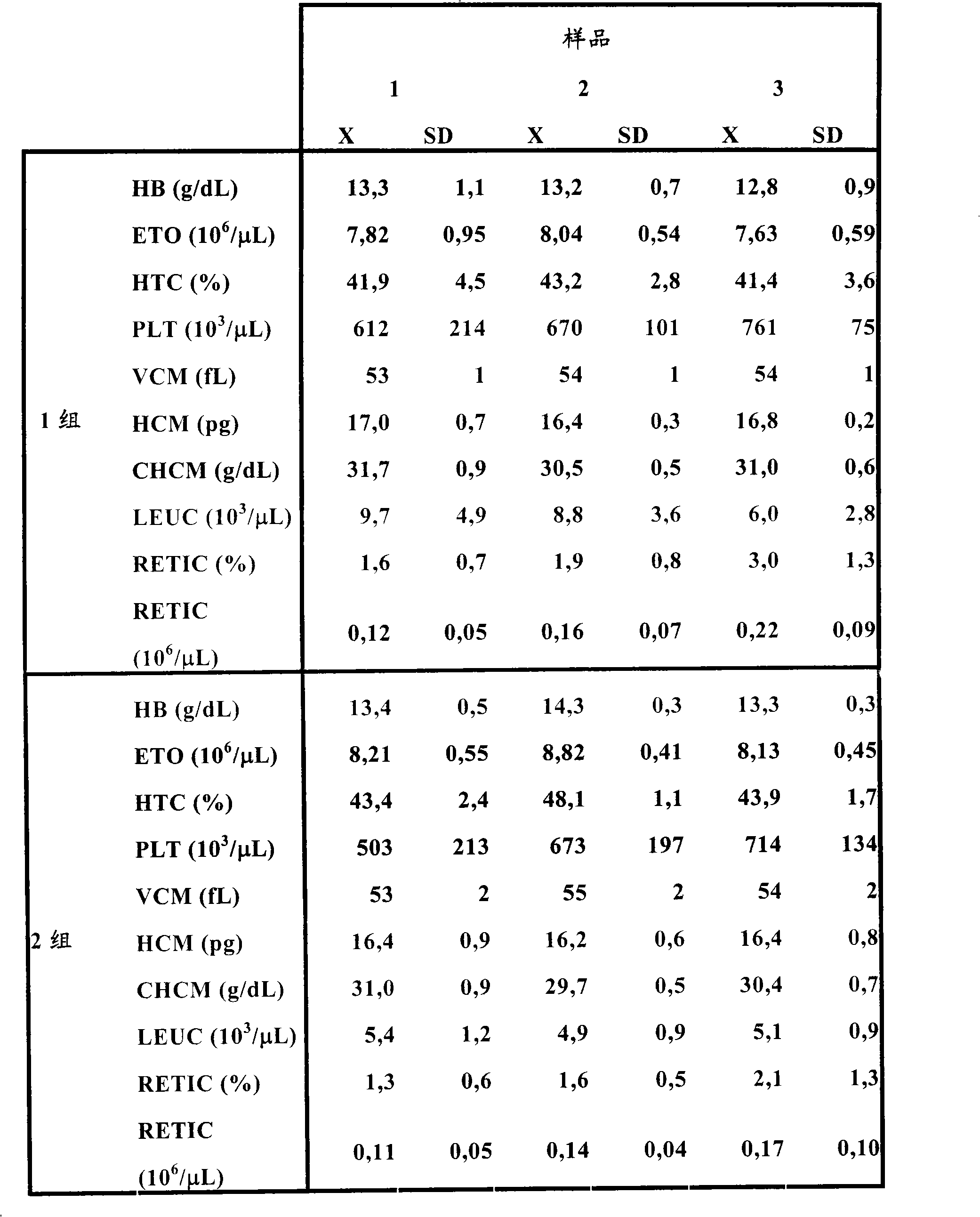
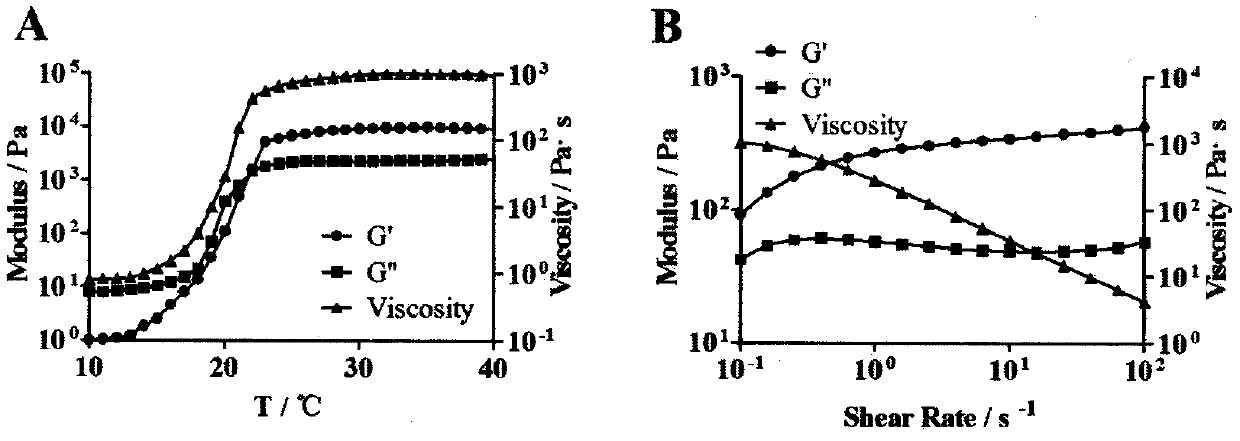
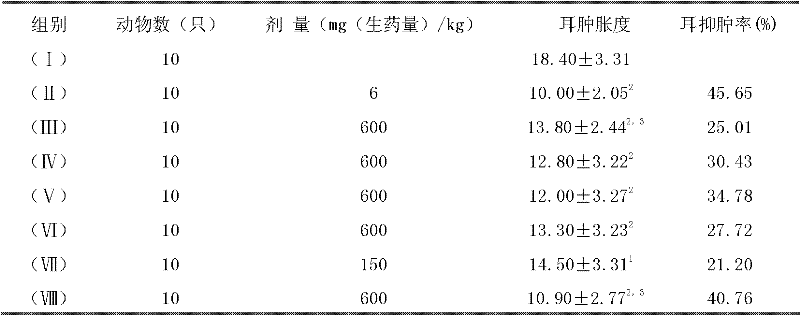
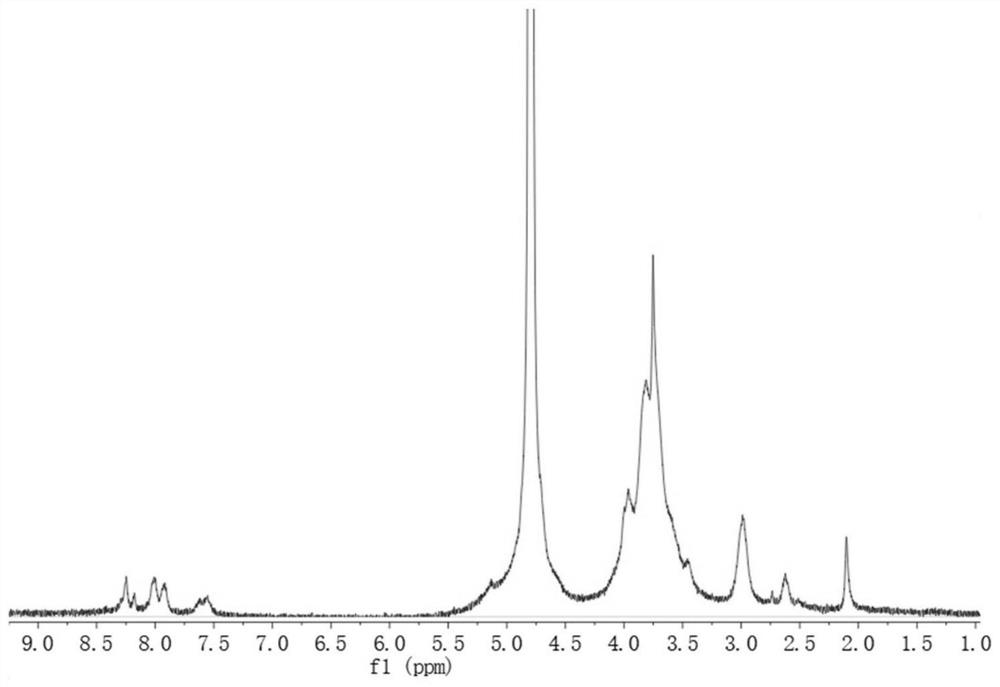
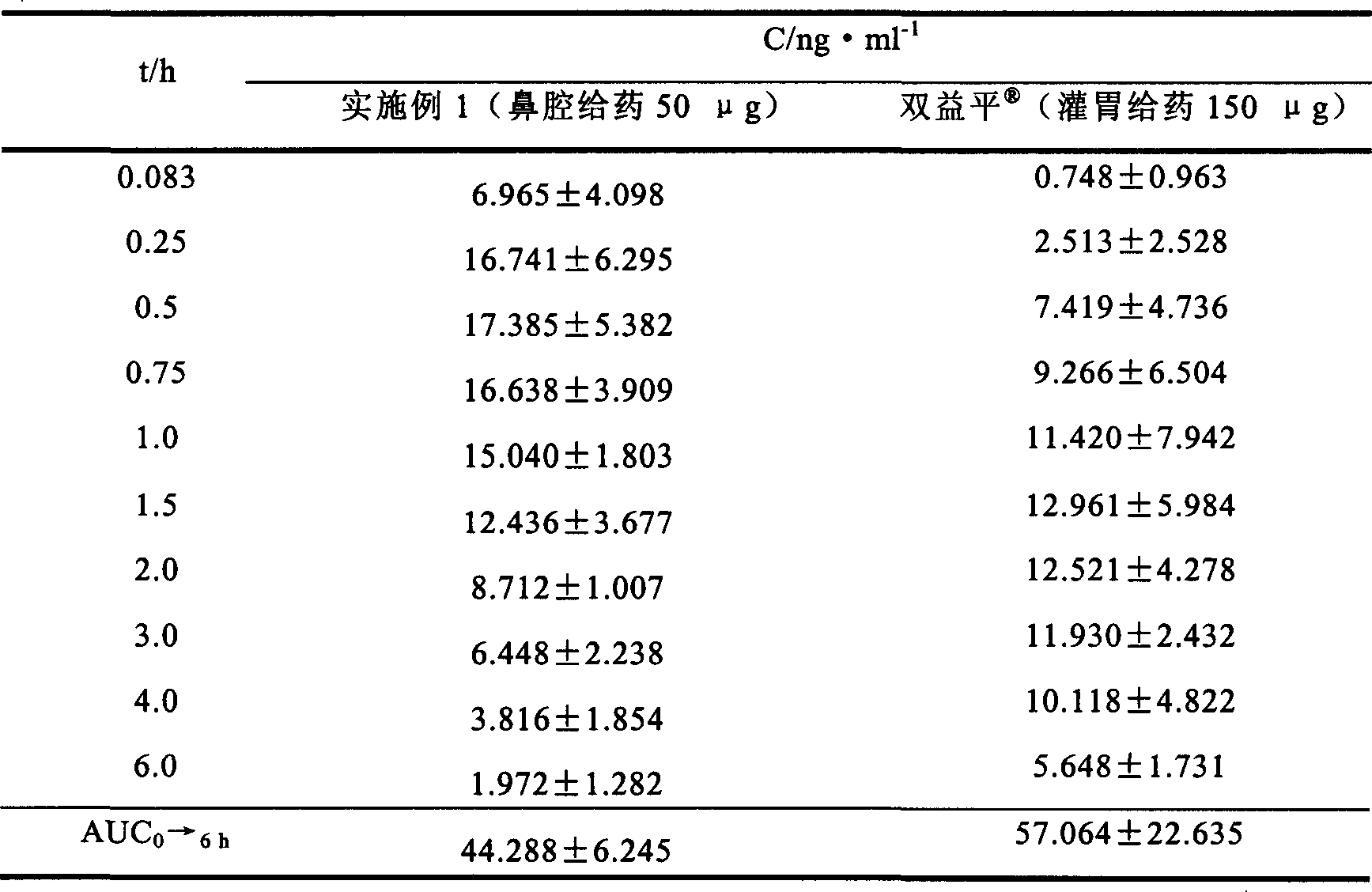
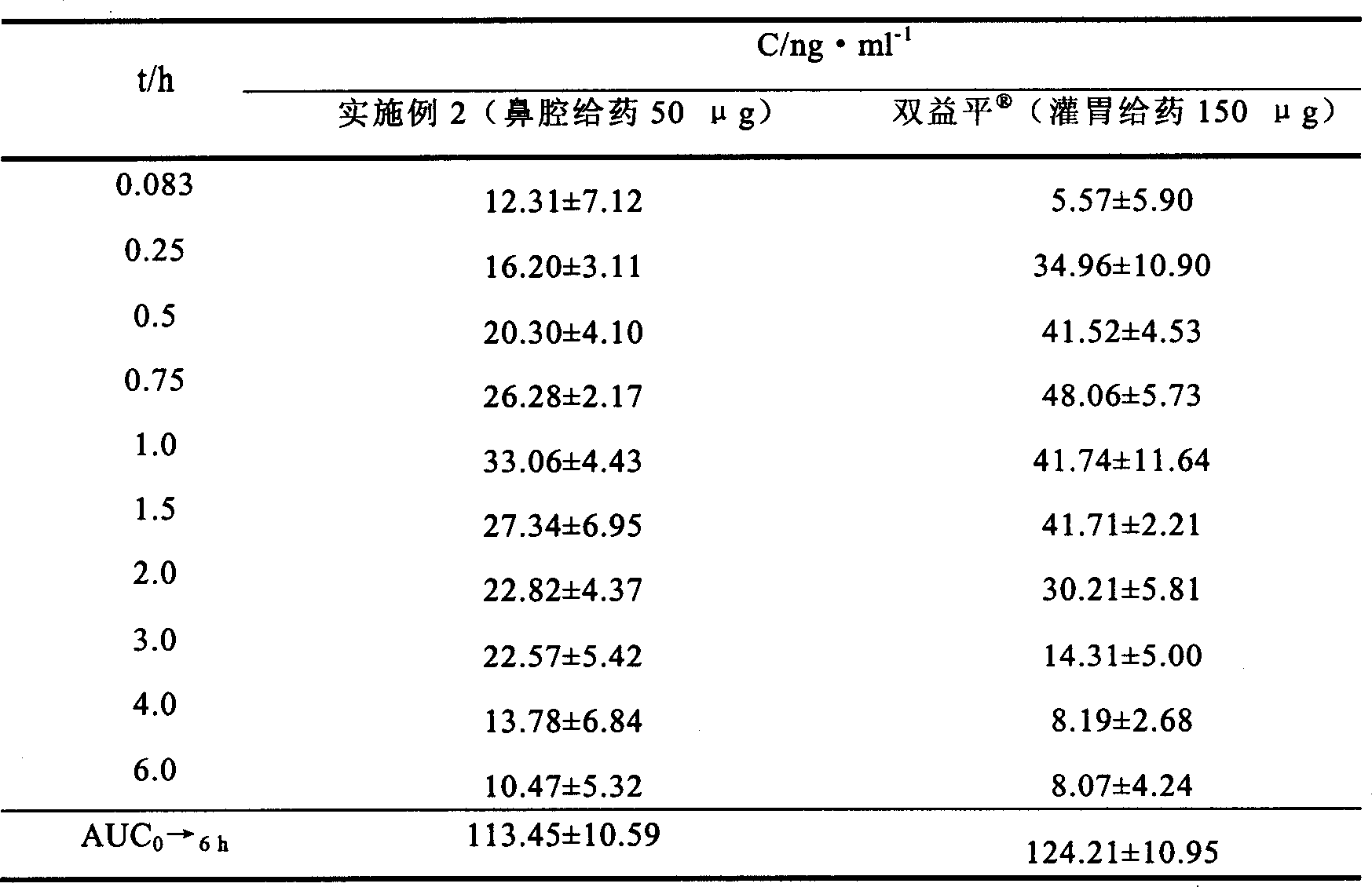
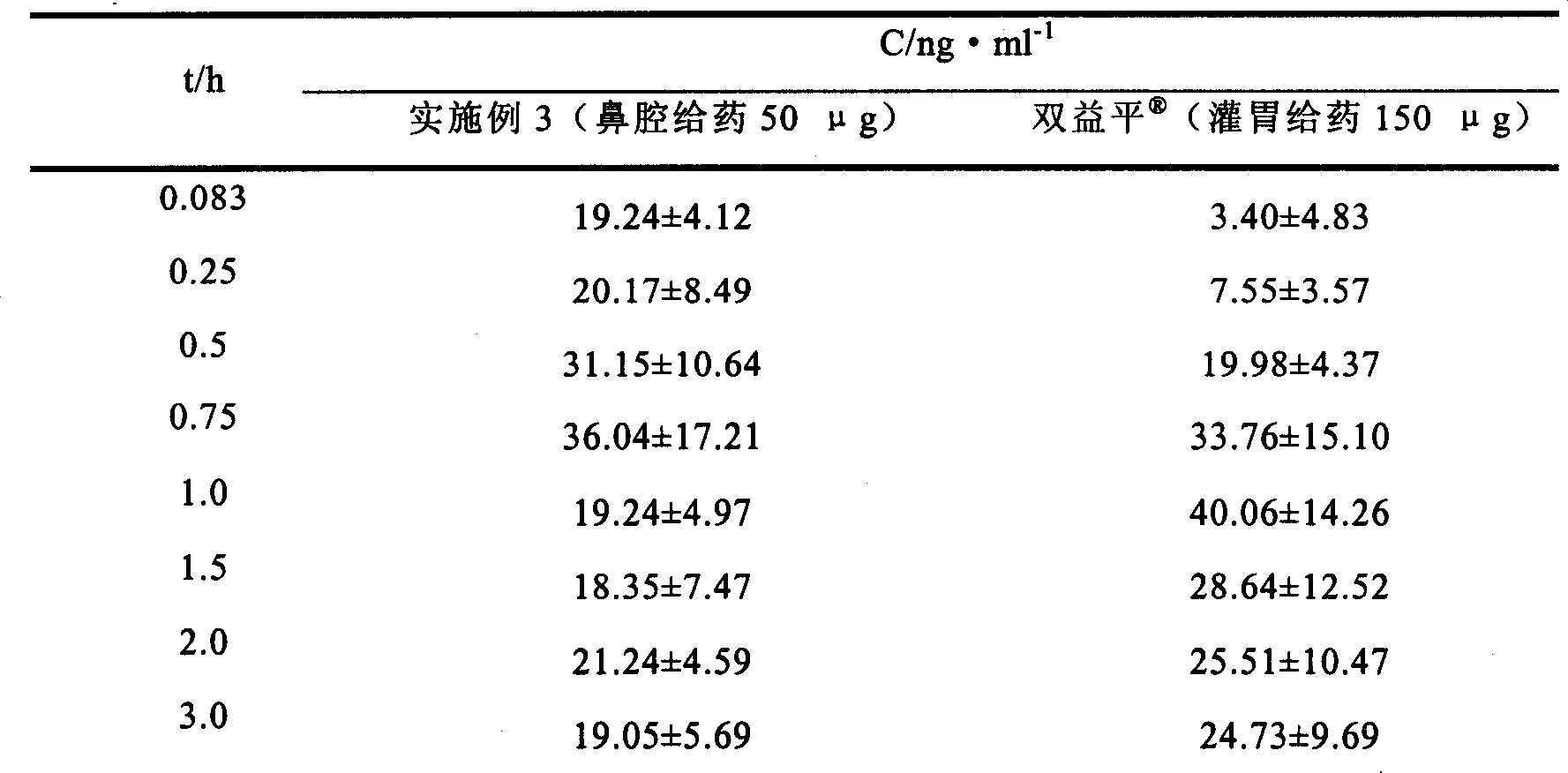
Patents
Literature
38 results about "NASAL PREPARATIONS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nasal preparations are products applied in the nose to treat conditions of the nose or nasal symptoms. They include medicines such as nasal steroids, lubricants, antihistamines and decongestants and anti-infectives, used to treat hay fever symptoms, congestion and infections. Nasal preparations are available as sprays, drops, creams, ointments and solutions for irrigation.
Nasal formulation
InactiveUS20070286813A1Improve nasal airflowBiocideInorganic active ingredientsNasal passageNasal cavity
The present invention relates to a novel nasal formulation for the treatment and prophylaxis of nasal congestion. When an infant is born, its nasal passages are relatively sterile, in time the nasal passages are colonized by fungi, molds and other organisms which compromise the integrity of the nasal and paranasal mucosa. The presence of these organisms elicits a mild chronic inflammatory reaction of the nasal and paranasal mucosa, in some cases leading to congestion and excess production of mucus. This chronic inflammatory process compromises the integrity of the nasal and paranasal mucosa, making it more vulnerable to binding with allergens and other invading organisms such as the rhinovirus. The purpose of this invention is to establish a treatment and prophylaxis of nasal congestion, which will control the presence of the invading organisms in the nasal and paranasal mucosa, reducing congestion as well as mucus production. By reducing the mild chronic inflammation in the nasal passages, prophylaxis with this nasal formulation passively reestablishes the integrity of the nasal and paranasal mucosa, thereby reinforcing the body's defenses against the implantation of allergens and of organisms such as the rhinovirus.
Owner:TOUTOUNGHI CAMILLE
Nasal spray formulation and method
InactiveUS20060008420A1Prevent signAvoid symptomsOrganic active ingredientsPeptide/protein ingredientsCyclodextrinNasal spray
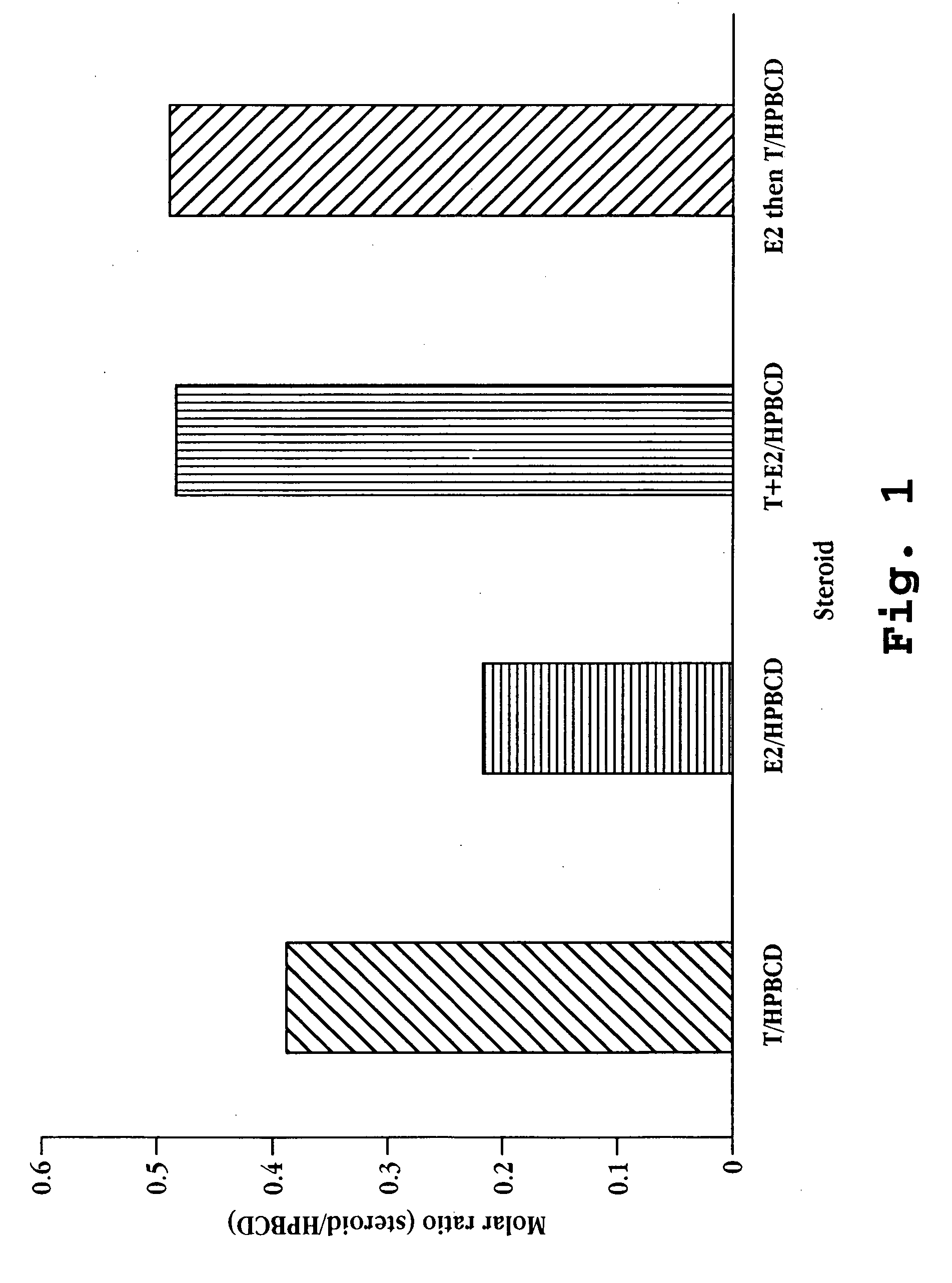
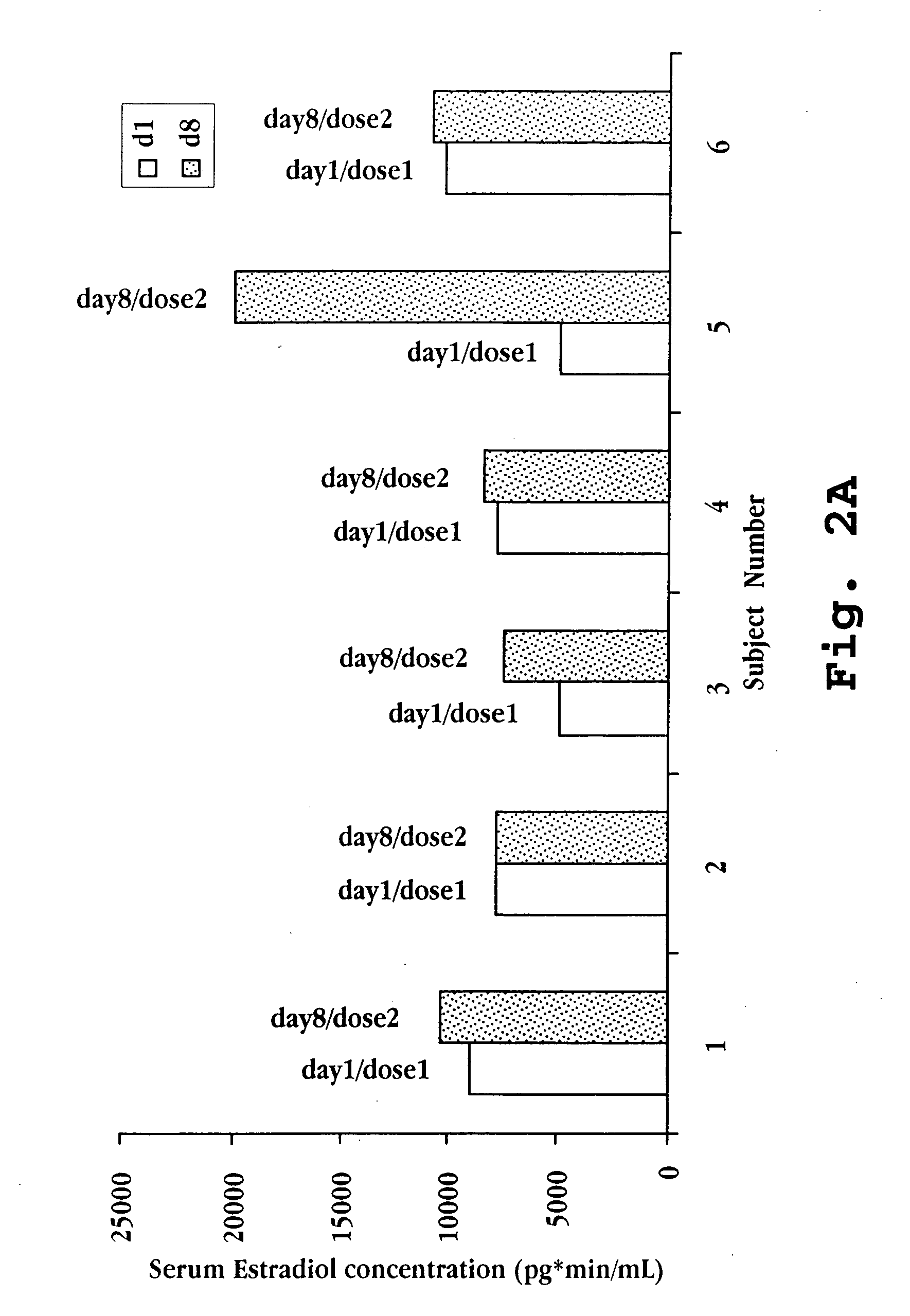
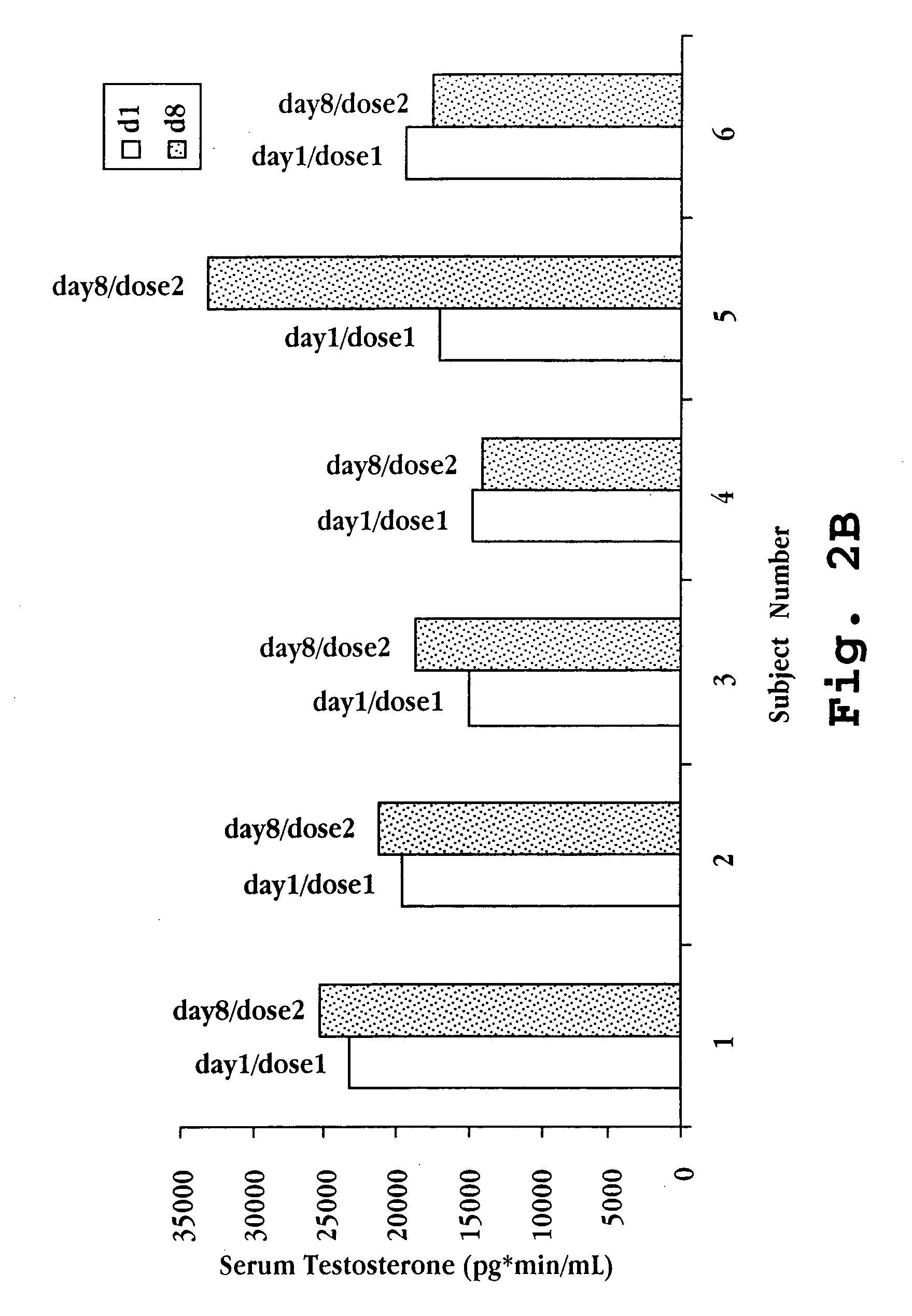
A nasal spray formulation for use in female contraception or in the treatment of benign gynecological disorders is described. The nasal preparation is comprised of a GnRH compound and an estrogenic compound in the form of a water-soluble complex with a water-soluble cyclodextrin. The preparation effectively suppresses ovarian estrogen and progesterone production, and prevents signs and symptoms of estrogen deficiency, without a significant increase in the risk of endometrial hyperplasia.
Owner:BALANCE PHARMA
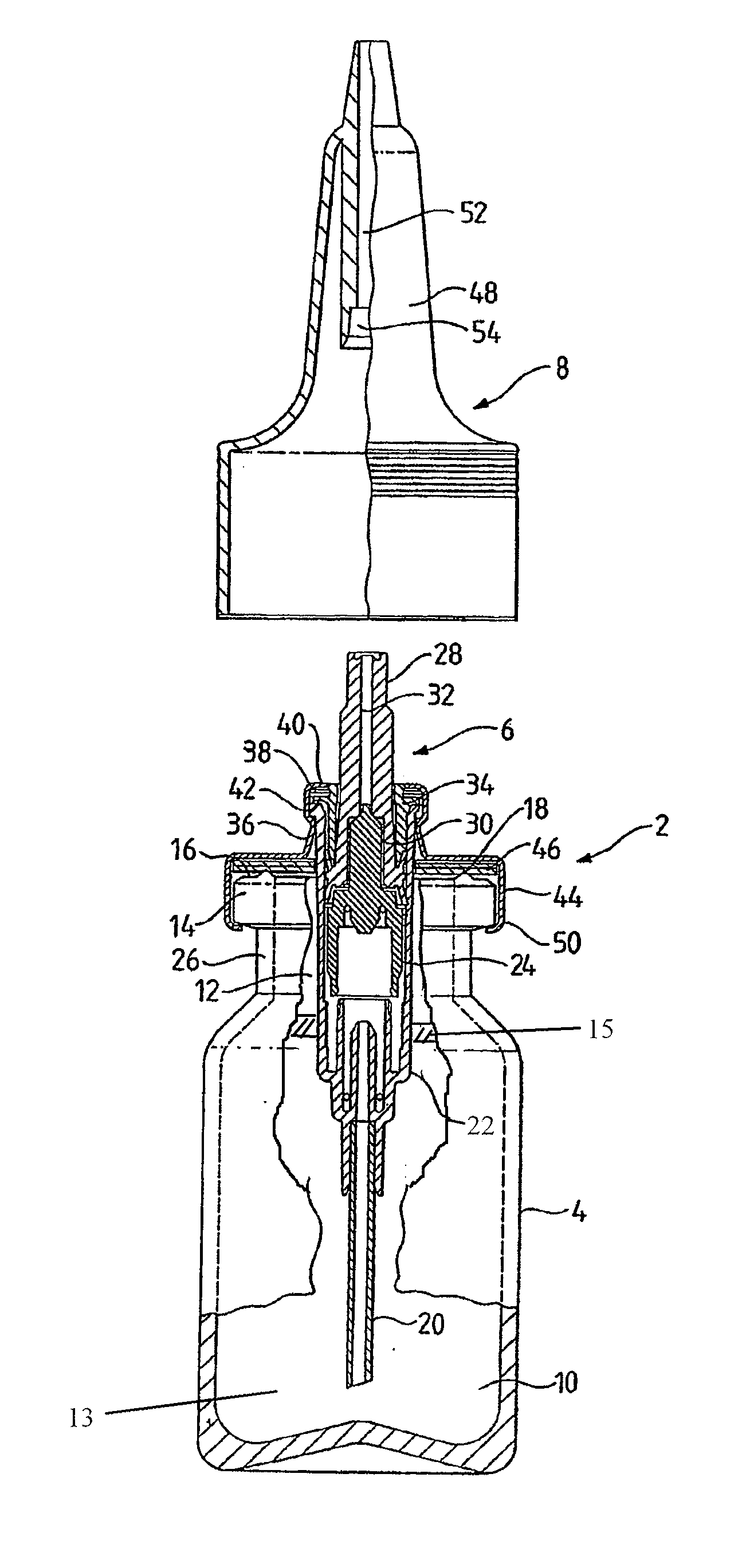
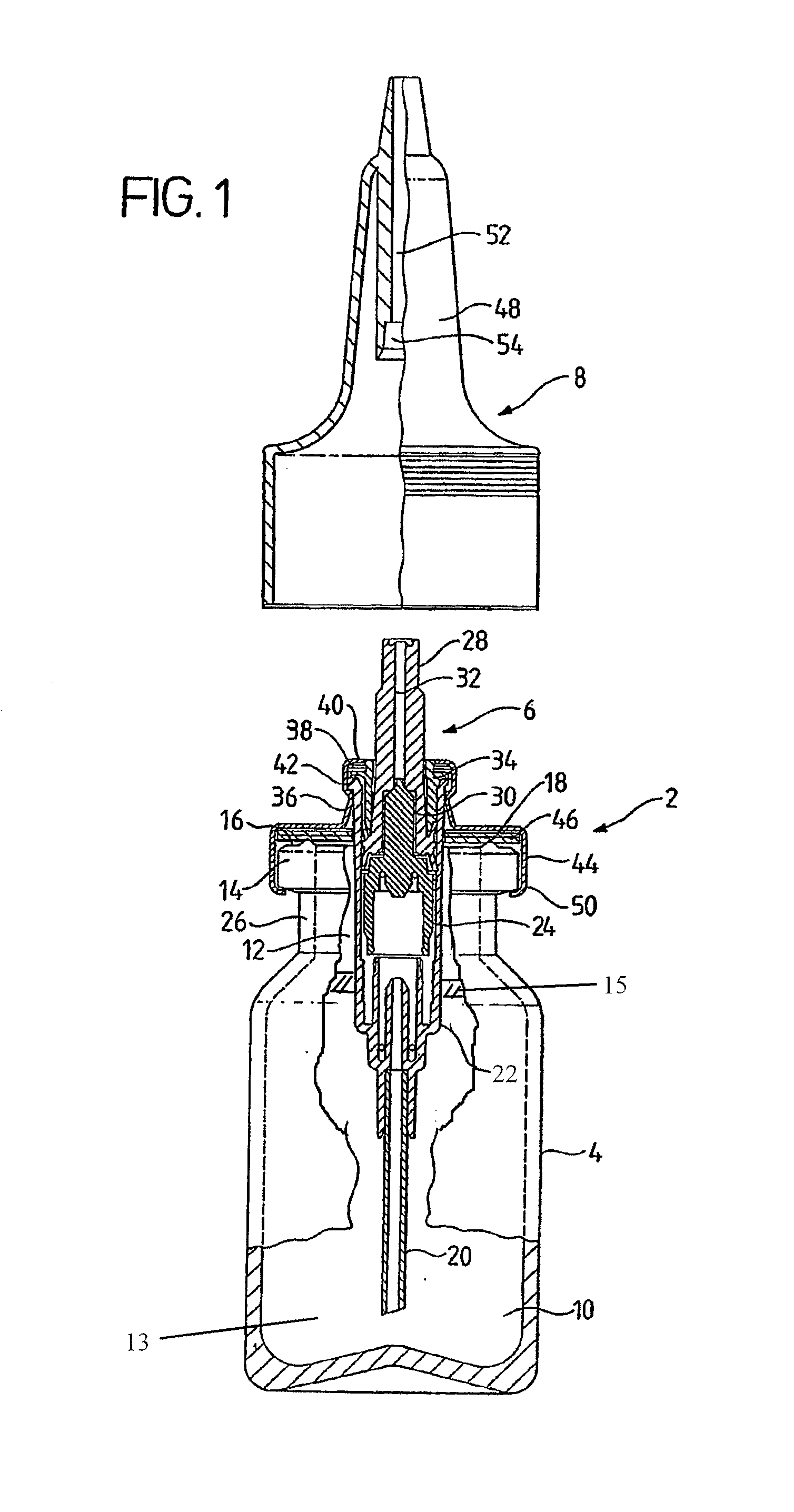
Nasal spray apparatus
A nasal spray comprises a container holding a nasal preparation containing sesame oil and a spray pump dispenser mounted to close an opening of the container. A dip tube from the dispenser extends down into the nasal preparation. The pump body is provided with a mounting collar operable to create an airtight seal with the top of the container. The nasal spray further comprises an inert substance covering a surface of the nasal preparation disposed within the container that is not contiguous with a wall of the container.
Owner:PHARMACURE HEALTH CARE
Nasal gel or ointment preparation for preventing and/or treating aspiration allergy
InactiveCN102764230AAvoid accessEliminate or relieve allergy symptomsAerosol deliveryOintment deliveryNasal cavityExtensibility
The invention relates to a nasal preparation for preventing and / or treating aspiration allergy. With white vaseline as a matrix, the nasal gel or ointment preparation provided by the invention contains coating-extensibility pharmaceutic adjuvants required for adjustment of the gel or ointment preparation. When the nasal gel or ointment is smeared on the inner wall of nasal cavity, a layer of protective film, like an invisible mouth mask, is formed in the nasal cavity, thus effectively blocking inhalant allergens (such as pollen in the air and the like) from entering human body through the nasal cavity. Thereby, allergy symptoms are eliminated or alleviated, and the nasal gel or ointment containing drug components further can simultaneously play the role of drug treatment as well.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Nasal formulation
InactiveUS20070286812A1Improve nasal airflowImproving nasalBiocideBacteria material medical ingredientsNasal cavityOral hygiene
The present invention relates to a novel nasal formulation for nasal hygiene and to improve nasal airflow of people suffering from nasal congestion.
Owner:TOUTOUNGHI CAMILLE
Curcumin microemulsion ion sensitive in situ gel preparation for intranasal administration and preparation method thereof
The invention discloses a curcumin microemulsion ion sensitive in situ gel preparation for intranasal administration, which uses curcumin as a raw medicine and adopts a surfactant, a cosurfactant, an oil phase and a gellan gum solution. According to the portions by weight, the preparation comprises 0.5-8 portions of curcumin, 4-25.6 portions of oil phase, 17-46.4 portions of surfactant, 17-46.4 portions of cosurfactant and 20-50 portions of gellan gum solution, wherein the mass concentration of the gellan gum solution is 0.1%-1.0%. The preparation method comprises the following steps of: firstly, uniformly mixing the oil phase, the surfactant and the cosurfactant in a mode of electromagnetic stirring, vortex oscillation or ultrasound; secondly, adding the curcumin, and fully stirring the mixture to be dissolved; thirdly, dropwise adding the gellan gum solution; and finally, continuously stirring the mixture to obtain the curcumin microemulsion ion sensitive in situ gel preparation for intranasal administration. After intranasal administration of the microemulsion ion sensitive in situ gel preparation disclosed by the invention, the medicine can reach brain targeted sites through a direct nose-brain path and a systemic circulation path, so that the curcumin has a treatment effect on brain tissues.
Owner:SHANDONG UNIV
Novel edaravone preparation and preparation method thereof
InactiveCN105878171AIncrease concentrationReduce concentrationOrganic active ingredientsNervous disorderDiseaseNasal cavity
The invention provides a preparation by which an edaravone drug can be targeted to a brain by virtue of a nasal delivery route, and a preparation method of the preparation. The edaravone nasal delivery preparation of the invention consists of an active ingredient, namely edaravone, and pharmaceutically acceptable effective adjuvant materials. The edaravone nasal preparation disclosed by the invention, after administration, can be rapidly absorbed and can rapidly reach the brain and enrich in the brain, so that a drug concentration in the brain, higher than that of an injection, which is administrated by an equivalent dosage, is provided, and a therapeutic effect on corresponding diseases is improved; meanwhile, by reducing edaravone concentrations in blood and other tissues, edaravone adverse reactions such as liver function damage and the like can be relieved; in addition, the compliance of patients can be improved; and the preparation is suitable for self-administration, so that the preparation, in the case of diseases, can be timely administrated as soon as possible.
Owner:NANJING HAIHENG MEDICINE SCI & TECH CO LTD
Pharmaceutical composition for nose administered in-situ gel spray of Huperzine A, preparation process and use thereof
InactiveCN1961879AIncrease brain selectivityEnhance memoryOrganic active ingredientsNervous disorderNasal cavityNose
The invention relates to a compound of original gel atomizer of Huperzine nose, and relative preparation, wherein it is external liquid, which can be atomized into nose as gel state. The invention use Huperzine as active component, assisted with original gel agent, pH adjuster, water, etc. The invention can treat senile dementia, and improve memory level of children. The invention has simple application, while it can be atomized into nose. And its cerebrospinal fluid and jordonet snyder utilization is 2.2-2.7 times of oral agent, and its effect is 2-4 times of oral agent, with low toxic.
Owner:SHANGHAI INST OF PHARMA IND
Amorphous Composition
InactiveUS20080096924A1Improve stabilitySmall individual differencesBiocideNervous disorderNasal cavityChemical stability
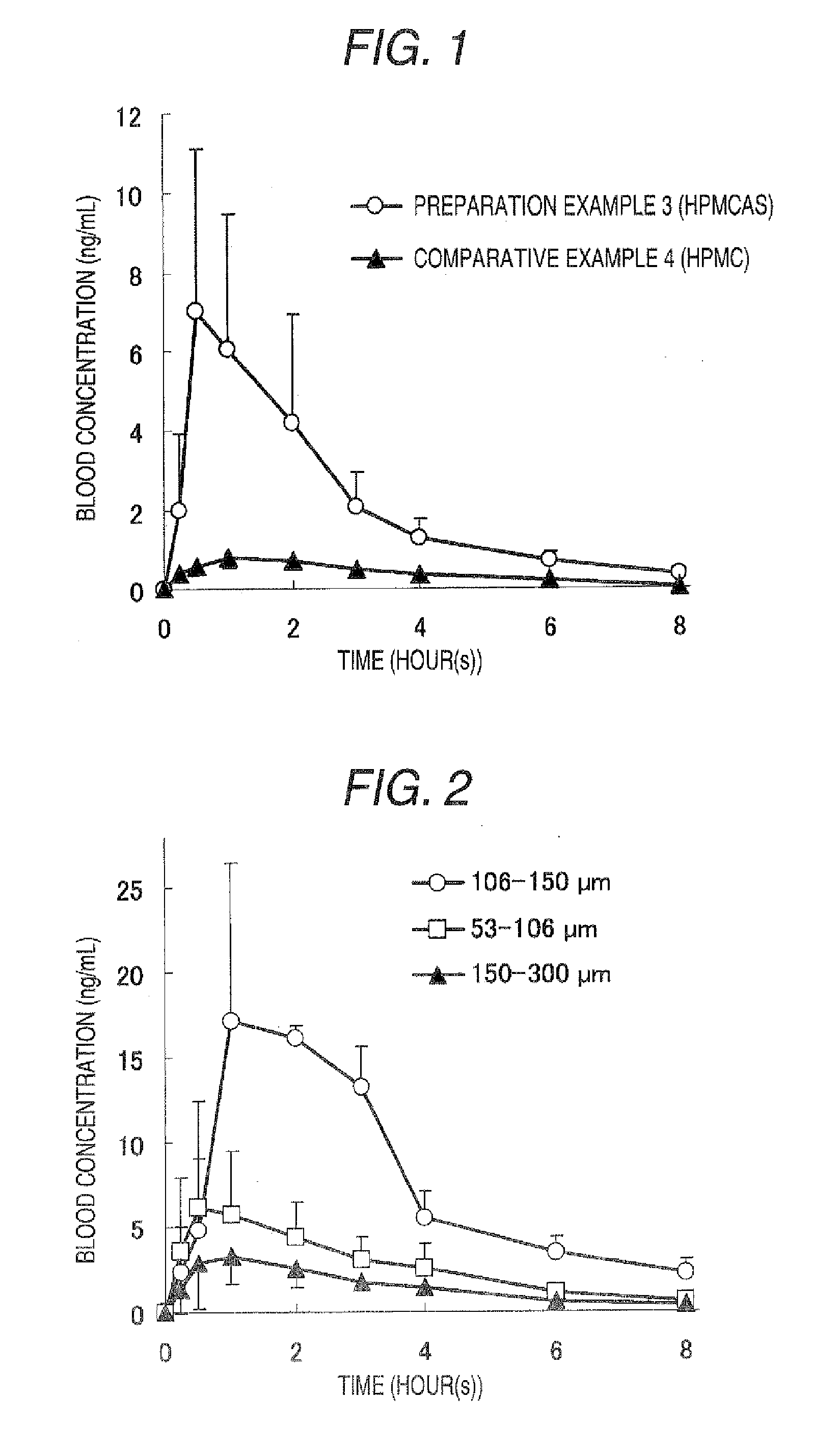
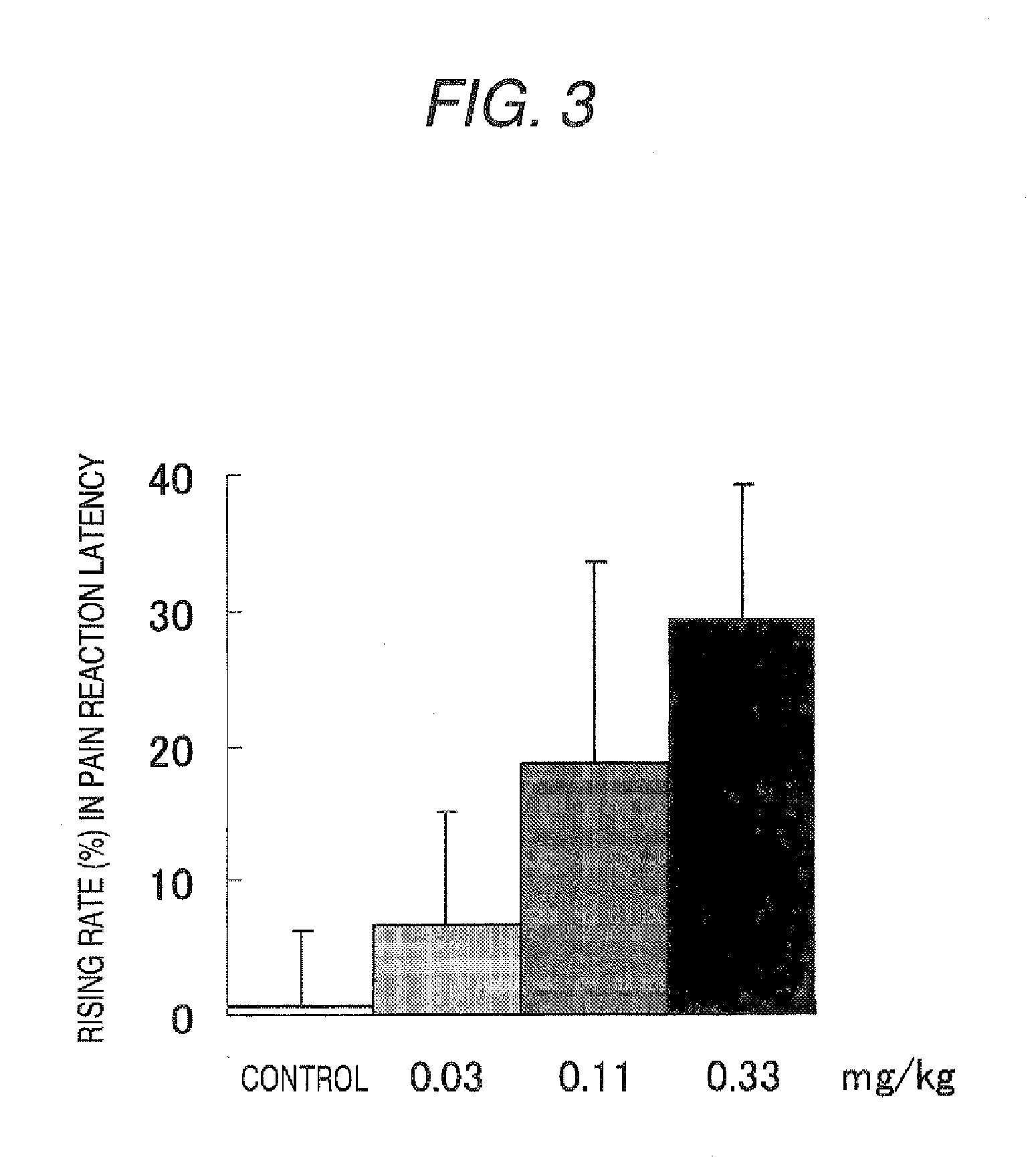
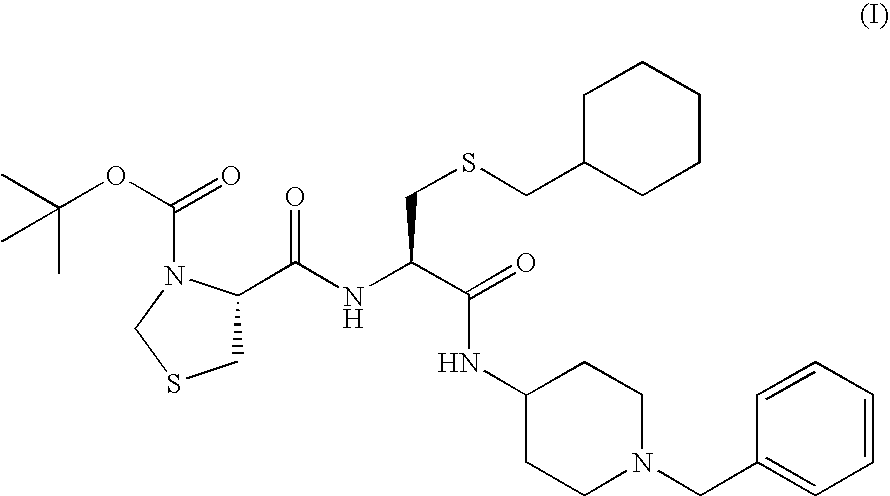
To provide an amorphous composition for nasal administration or for administration by adhering to oral mucosa in which absorption property and chemical and physical stabilities of (2R)—N-(1-benzylpiperidin-4-yl)-3-cyclohexylmethylthio-2-[(4R)-3-tert-butoxycarbonylthiazolidin-4-ylcarbonylamino]propanamide which is useful as an N type calcium channel inhibitor are improved. A preparation comprising the amorphous composition of the present invention has been found to be excellent in physical stability and chemical stability and to be useful as a nasal preparation or a preparation for adhering to the oral mucosa. As a result, the resulting preparation has a high BA value and is useful for prevention and / or the treatment of a disease mediated by the N type calcium channel including pain (such as neuropathic pain, cancerous pain, intractable pain, postoperative pain, acute pain, chronic pain, neuralgia and infectious pain).
Owner:ONO PHARMA CO LTD
Herba houttuyniae volatile oil liposome preparation and preparation method and usage thereof
InactiveCN108403834APromote enrichmentImprove securityRespiratory disorderLiposomal deliveryWhole bodyHouttuynia
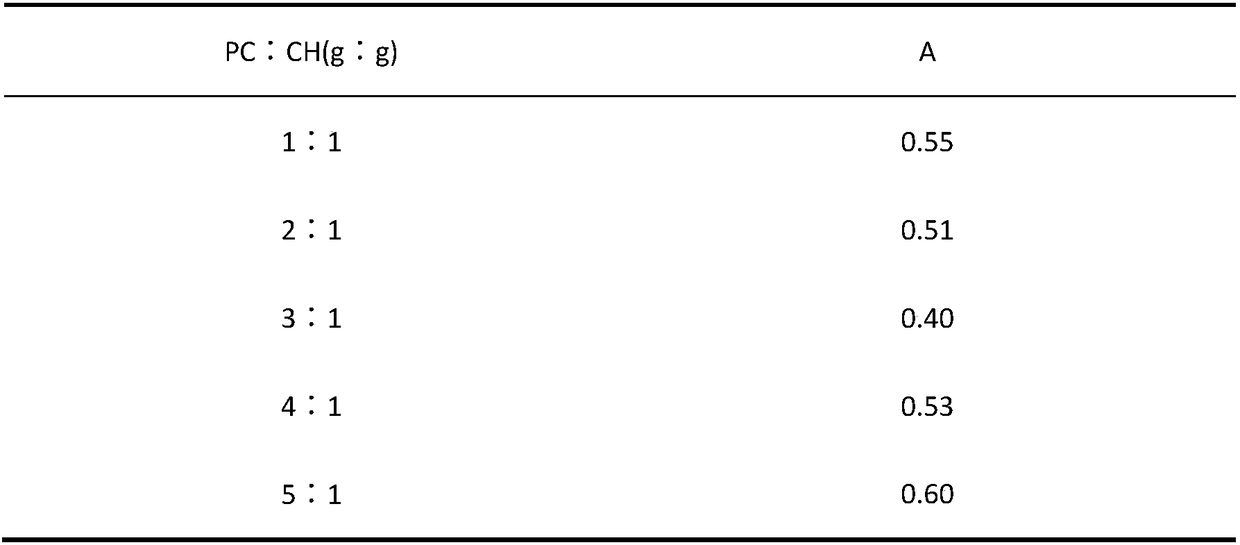
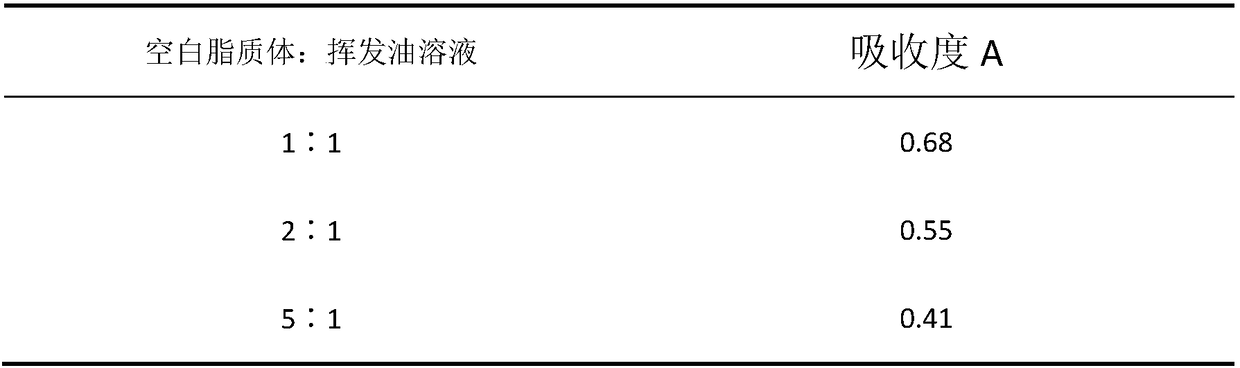
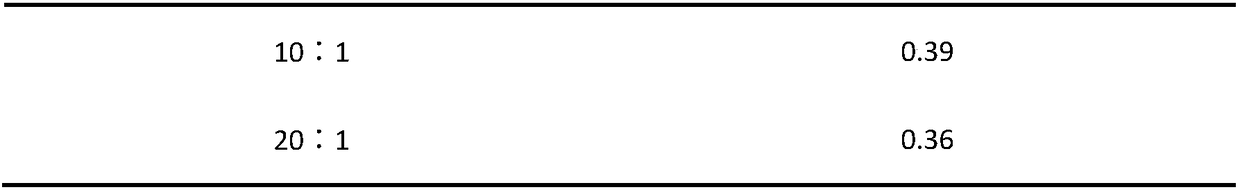
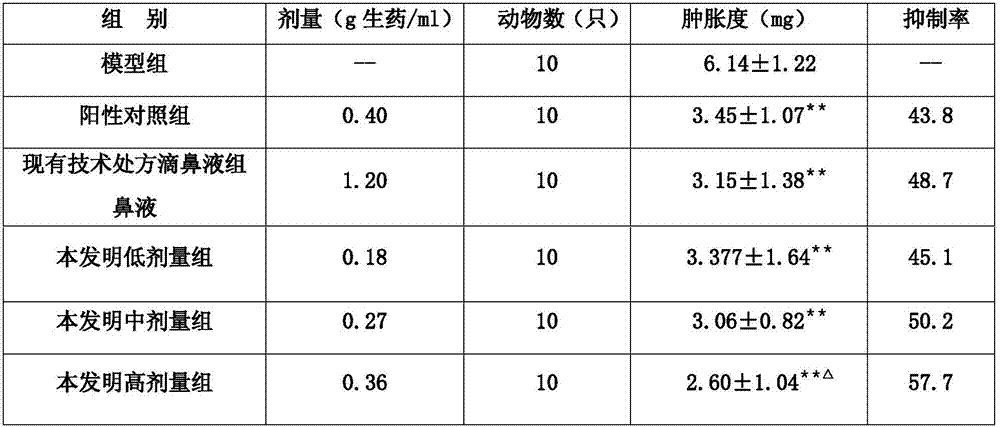
The invention provides a herba houttuyniae volatile oil liposome preparation. The herba houttuyniae volatile oil liposome preparation contains liposome formed by phospholipid and cholesterol and herbahouttuyniae volatile oil encapsulated by the liposome, wherein a weight ratio of the phospholipid and cholesterol is 2-4:1, and a weight ratio of the liposome and the herba houttuyniae volatile oil is 5:1. The herba houttuyniae volatile oil liposome can be used for preparing a nasal preparation, the safety is greatly improved, and the curative effect is good. The herba houttuyniae volatile oil liposome can be enriched in a respiratory tract better after absorbed by a nose so that the good local action is achieved, and absorbed into the blood circulation through respiratory region mucous membranes, the whole body therapeutic action is achieved, so the preparation has the good clinic application prospect.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Nasal preparation for treating rhinitis and its preparation method and application
ActiveCN107412331AImprove complianceGood effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSide effectAdditive ingredient
The invention relates to the technical field of nasal preparation, and relates to a nasal preparation for treating rhinitis and its preparation method and application. The nasal preparation for treating rhinitis is prepared from, by weight, artemisia rupestris, fried cocklebur fruit, radix angelicae, ephedra, scutellaria baicalensis extract, extractum glycyrrhizae, dementholized peppermint oil, tween-80, and ingredients. The nasal preparation for treating rhinitis is prepared by combining ethnic medicine theory with traditional Chinese medicine theory; compared with the prior art, the dosage of the nasal preparation for treating rhinitis is only 20-30% of that in the prior art; moreover, the nasal preparation for treating rhinitis has significant effects in anti-inflammatory and antiallergic by comparing with the prior art; meanwhile, the nasal preparation is simple in preparation technique, accurate in treatment effect, free from toxic and side effects; the drug is directly effected on the surface of the nasal mucosa; thus the nasal preparation is high in bioavailability, quick and convenient to take effect and good in patient compliance; different dosage forms can be prepared upon patient's demand, thus the nasal preparation can be better applied to treat acute, chronic and allergic rhinitis.
Owner:XINJIANG INST OF MATERIA MEDICA
Cannabidiol nasal preparation for treating post-traumatic stress disorder
The invention belongs to the field of pharmaceutical preparations, and particularly relates to preparation of a cannabidiol nasal preparation composition for treating post-traumatic stress disorder, and temperature sensitive gel, ion sensitive gel, cubic liquid crystals, and the like are included; the cannabidiol nasal preparation composition has high adhesion in a nasal cavity, residence time isprolonged in the nasal cavity, bioavailability is improved beneficially to exert the pharmacological effect, and brain targeting is achieved; at the same time, the preparation process is simple, stability is high, and compliance of the patient is good; and pharmacodynamic tests prove that the cannabidiol nasal preparation for treating the post-traumatic stress disorder has good anti-depressant andanti-anxiety effects, and can be used for treating the post-traumatic stress disorder.
Owner:ACADEMY OF MILITARY MEDICAL SCI
A medicament for treating cerebrovascular disease
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Probiotic composition for preventing and treating allergic rhinitis and nasal preparation based on probiotic composition and preparation method of nasal preparation
ActiveCN109512854AGood effectProlong survival timeAerosol deliveryOintment deliveryNasal cavitySide effect
The invention discloses a probiotic composition for preventing and treating allergic rhinitis and a nasal preparation based on the probiotic composition and a preparation method of the nasal preparation. Probiotics are used as an active ingredient, and a composite stabilizer is used as a matrix. Nasal gel or ointment of the nasal preparation is applied to the inner wall of a nasal cavity to form aprotective film in the nasal cavity, just like an invisible mask is worn, the inhaled allergens (such as pollen in the air) are effectively prevented from entering a human body through the nasal cavity, and allergic symptoms are eliminated or alleviated; and meanwhile, the contained probiotic composition can exert an immunomodulatory effect more rapidly and directly on the nasal mucosa, that is,by promoting the increased secretion of interleukin 12 (IL-12) and interferon gamma, Th1-type immune response is regulated to inhibit immune globulin IgE, an allergic phenomenon of Th2-type immune overreaction is improved, and other side effects are not caused.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Nasal liquid medicine for treating headache and its prepn process
InactiveCN1973847AImprove absorption rateHigh yieldNervous disorderAerosol deliveryNasal InhalantNose
The present invention is one kind of nasal liquid medicine for treating headache and its preparation process. The medicine consists of volatile dahurian angelica oil and volatile Chuanxiong oil as the effective medicine components and pharmaceutically acceptable supplementary material. The medicine is prepared into emulsion form or gel form, such as nasal drop, nasal spray, aerosol, nasal inhalant, etc. The volatile oil as the effective component is preferably extracted through supercritical CO2 circular extraction and separation. The medicine for treating headache has high bioavailability and excellent effect.
Owner:成都厚发科技开发有限公司
Treatment of symptoms associated with female gastroparesis
InactiveUS20190388370A1Improve the quality of lifeOrganic active ingredientsDigestive systemGastroparesisMetoclopramide
Nasal formulations of metoclopramide are administered for the treatment of symptoms associated with female gastroparesis. Also provided are methods of treating symptoms of female gastroparesis with nasal metoclopramide.
Owner:EVOKE PHARMA INC
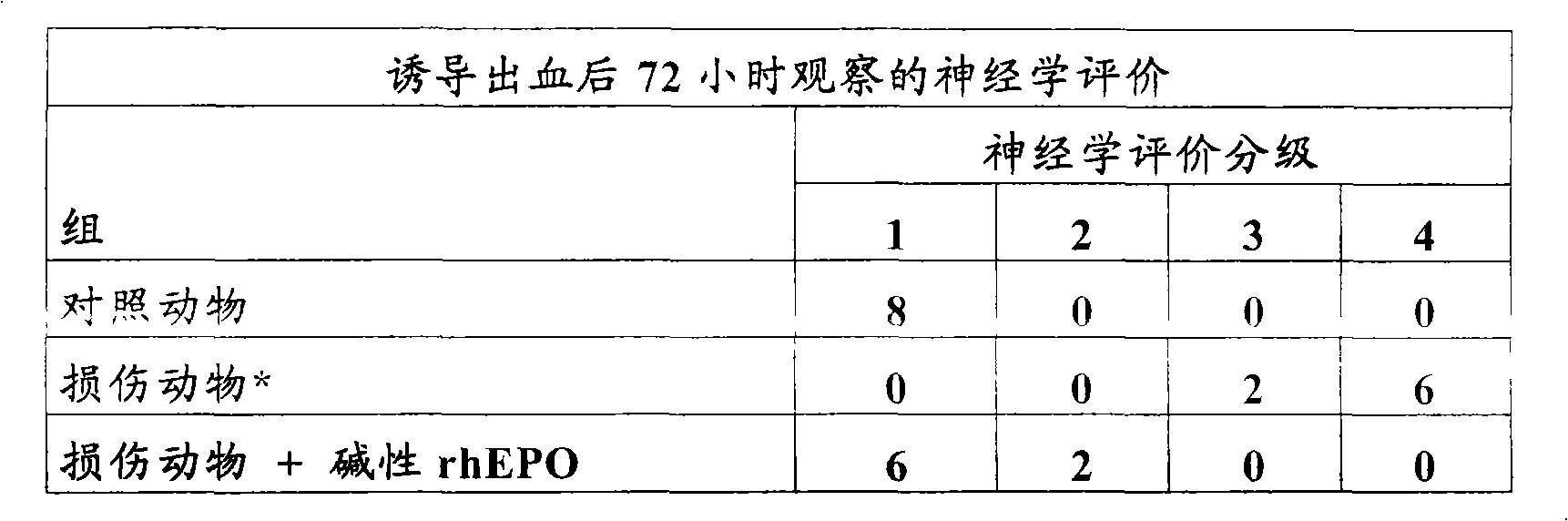
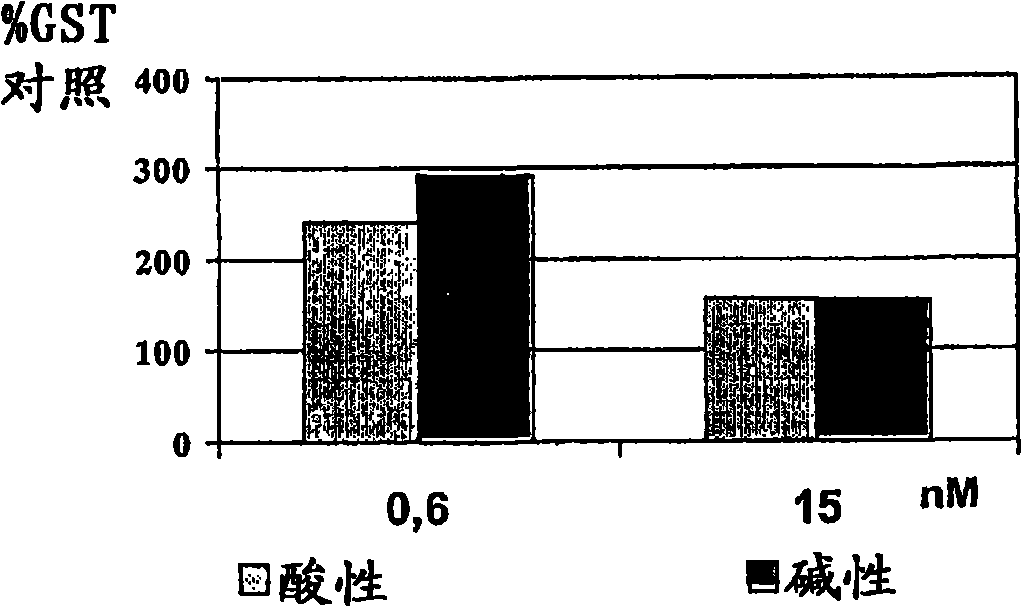
Rh-epo nasal formulations with low sialic acid concentration for the treatment of diseases of the central nervous system
The invention relates to the biopharmaceutical industry and, in particular, to the development of a medicament which is intended for the treatment of cerebrovascular, neurodegenerative and psychiatric diseases and which contains, by way of active principle, recombinant human erythropoietin (rh-Epo) with a low sialic acid concentration. During rh-Epo production, the glycoprotein is obtained with a different sialic acid concentration when less than 40 % of the molecule thereof is protected with (basic) sialic acid, in which case it is not biologically active by systemic route and is inactivated by hepatic enzymes. Surprisingly, it was found that nasally-administered basic rh-Epo has greater therapeutic benefits than the acid.; The inventive basic rh-Epo nasal formulations include bioadhesive polymers which increase the residence time in the nasal cavity, thereby enhancing the therapeutic effect thereof. Said formulations also include other auxiliary substances, such as preservative substances, surfactants, pH regulators, isotonising agents and protein stabilisers.
Owner:CENT DE INVESTIGACION & DESARROLLO DE MEDICAMENTOS CIDEM
Nasal liquid medicine for treating headache and its preparation process
InactiveCN100536860CImprove absorption rateHigh yieldNervous disorderAerosol deliveryEmulsionNasal Inhalant
The present invention is one kind of nasal liquid medicine for treating headache and its preparation process. The medicine consists of volatile dahurian angelica oil and volatile Chuanxiong oil as the effective medicine components and pharmaceutically acceptable supplementary material. The medicine is prepared into emulsion form or gel form, such as nasal drop, nasal spray, aerosol, nasal inhalant, etc. The volatile oil as the effective component is preferably extracted through supercritical CO2 circular extraction and separation. The medicine for treating headache has high bioavailability and excellent effect.
Owner:成都厚发科技开发有限公司
Novel coronavirus antibody with broad-spectrum neutralizing activity as well as preparation method and application of novel coronavirus antibody
ActiveCN113999321AImprove thermal stabilityGood crossPolypeptide with localisation/targeting motifEgg immunoglobulinsNasal cavityViral antibody
The invention discloses a novel coronavirus antibody with broad-spectrum neutralizing activity as well as a preparation method and application of the novel coronavirus antibody. The preparation method of the novel coronavirus antibody comprises the following steps: immunizing a poultry animal by adopting an immunogen to obtain the antibody; wherein the immunogen comprises a protein A and a protein B, and the amino acid sequence of the protein A is shown as a sequence 3 in a sequence table; and the amino acid sequence of the protein B is shown as a sequence 6 in the sequence table. The novel coronavirus antibody has good cross-neutralization activity on wild strains and variant strains of the novel coronavirus, and the titer of the induced antibody is far higher than that of the reported antibody obtained by adopting an inactivated vaccine. After being inhaled by the nasal cavity of a mouse, the mouse can be protected against high-dose new coronavirus challenge, and no obvious pathological change is seen in the lung. After a big-ear white rabbit inhales in the nasal cavity, a high-titer neutralizing antibody can be detected in bronchus and lungs. The antibodies can be developed into nasal formulations for use in the prevention and / or treatment of new coronavirus infection.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Tetrandrine nasal preparation for treating post-traumatic stress disorder
ActiveCN110433132AEasy to wrapStrong bioadhesionOrganic active ingredientsNervous disorderNoseTetrandrine
The invention discloses a tetrandrine nasal preparation for treating post-traumatic stress disorder. Tetrandrine is prepared into temperature sensitive gel, a cubic liquid crystal and other nasal preparations, and the effect is good in treating the post-traumatic stress disorder through brain targeting.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Qingpeng nasal preparation for stopping pain and diminishing swelling and preparation method thereof
The invention discloses a Qingpeng nasal preparation for stopping pain and diminishing swelling, which is prepared by taking the prescription of traditional Tibetan medicine of Qingpeng ointment as a basic prescription and changing a formulation, and a preparation method thereof. The Qingpeng nasal preparation is prepared by a method that particle medicinal materials are extracted and partial medicinal materials are subjected to ultra-fine crushing, effectively maintains active ingredients of the medicinal materials, greatly improves the content of crude medicines, and improves bioavailability to make treatment effect better. Auxiliary materials selected have good mouldability, so that the product has good permeability, and is better to absorb and quick in response. The Qingpeng nasal preparation has the advantages of convenience of administration, long duration, low allergy rate, convenience of use, portability, no pollution to clothes and the like. A nasal mucosal medicine delivery system is adopted, is quick in absorption and response, and does not have any stimulation or odor. Pharmacodynamic experiments prove that the Qingpeng powder or granules have obvious effects of stopping pain and diminishing swelling.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Chitosan lipoprotein nasal delivery nanocomposite as well as preparation method and application thereof
ActiveCN113116854ASmooth and round appearanceGood dispersionMaterial nanotechnologyPowder deliveryA lipoproteinBrain targeting
The invention belongs to the field of pharmaceutical preparations, and discloses a chitosan lipoprotein nasal delivery nanocomposite. The chitosan lipoprotein nasal delivery nanocomposite comprises lipoprotein nanoparticles and chitosan or / and chitosan derivatives with a nasal mucosa absorption promoting effect. The invention also discloses a preparation method of the chitosan lipoprotein nasal delivery nanocomposite. The preparation method comprises the following steps of self-assembling the chitosan or / and the chitosan derivative and the lipoprotein nanoparticles into a nasal preparation with good membrane permeability through dynamic acting force. The chitosan lipoprotein nasal delivery nanocomposite is mild in preparation condition, simple in process and easy for industrial production. The system is safe and non-toxic, the biodegradability is good, the nasal mucosa absorption of lipoprotein drugs can be improved, and the efficient brain targeting property is ensured. The dosage form is administrated in a nasal dripping mode, a spraying mode and the like, operation is easy, patients taking medicine for a long time can take the medicine conveniently, and good clinical application prospects are achieved in the aspect of treatment of central nervous system diseases.
Owner:CHINA PHARM UNIV
Gastrodin nasal starch microspheres with bioadhesive properties and preparation method thereof
ActiveCN104971047BHigh encapsulation efficiencyHigh drug loadingOrganic active ingredientsNervous disorderOil phaseEthyl acetate
The invention provides a gastrodin nasal starch microsphere with bioadhesive properties. The microsphere is prepared by the following method: using starch sodium hydroxide aqueous solution as the water phase; dispersing gastrodin in ethyl acetate to obtain Oil phase; slowly add the oil phase to the water phase at room temperature, and stir in the same direction to obtain O / W colostrum; heat the liquid paraffin containing Span80 to 40-50°C, add the above colostrum into it, and stir in the same direction to emulsify After 20-40 minutes, add the cross-linking agent epichlorohydrin, cross-link at 30-50°C for 3-5 hours, centrifuge, pour off the upper oil phase, and wash the lower layer of microspheres with ethyl acetate, absolute ethanol, and acetone respectively Finally, dry under reduced pressure to obtain the described gastrodin nasal starch microspheres; the gastrodin nasal starch microspheres prepared by the present invention have a long retention time in the nasal cavity, and the drug release speed is stable and complete, which can improve the drug distribution in the brain, biological Utilization and efficacy, and safe and non-toxic.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
A kind of chitosan lipoprotein nasal administration nanocomposite and its preparation method and application
ActiveCN113116854BSmooth and round appearanceGood dispersionPowder deliveryMaterial nanotechnologyA lipoproteinPharmaceutical formulation
The invention belongs to the field of pharmaceutical preparations, and discloses a chitosan lipoprotein intranasal administration nanocomposite, comprising lipoprotein nanoparticles, chitosan or / and chitosan derivatives having the effect of promoting absorption of nasal mucosa. The invention also discloses a preparation method of chitosan lipoprotein intranasal administration nanocomposite, comprising: self-assembling chitosan or / and chitosan derivatives and lipoprotein nanoparticles through dynamic force to form a good membrane permeability nasal preparations. The preparation condition of the chitosan lipoprotein intranasal administration nanocomposite is mild, the process is simple, and the industrialized production is easy. The system is safe, non-toxic, and has good biodegradability, which can improve the absorption of lipoprotein drugs in the nasal mucosa and ensure high-efficiency brain targeting. The dosage form is administered by nasal drops, sprays, etc., and is easy to operate, convenient for patients who take the medicine for a long time, and has a good clinical application prospect in the treatment of central nervous system diseases.
Owner:CHINA PHARM UNIV
Nasal preparation for treating epilepsy
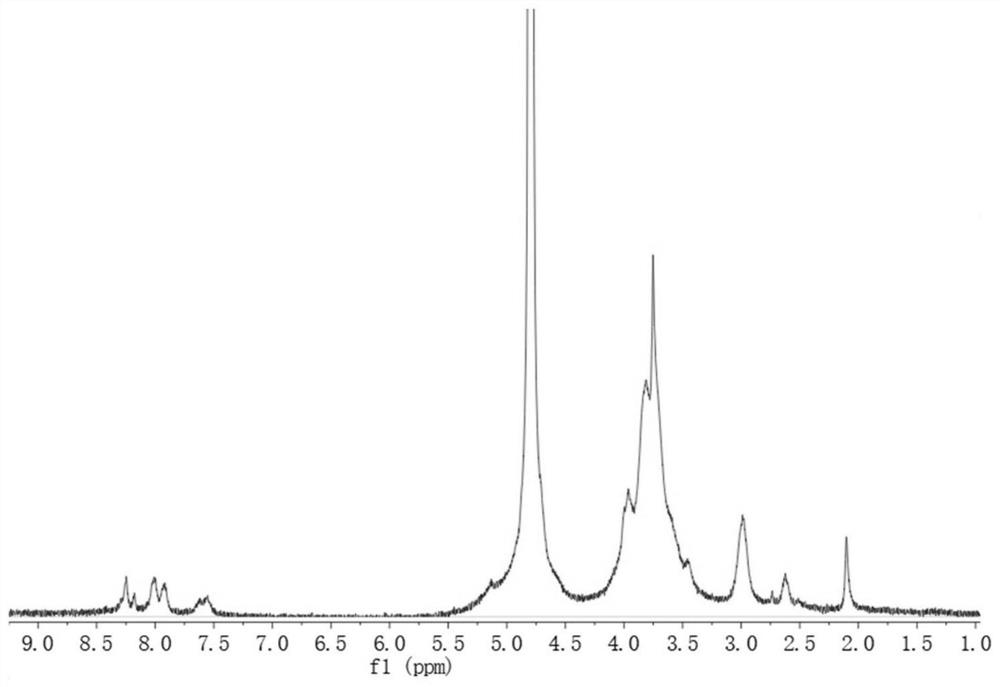
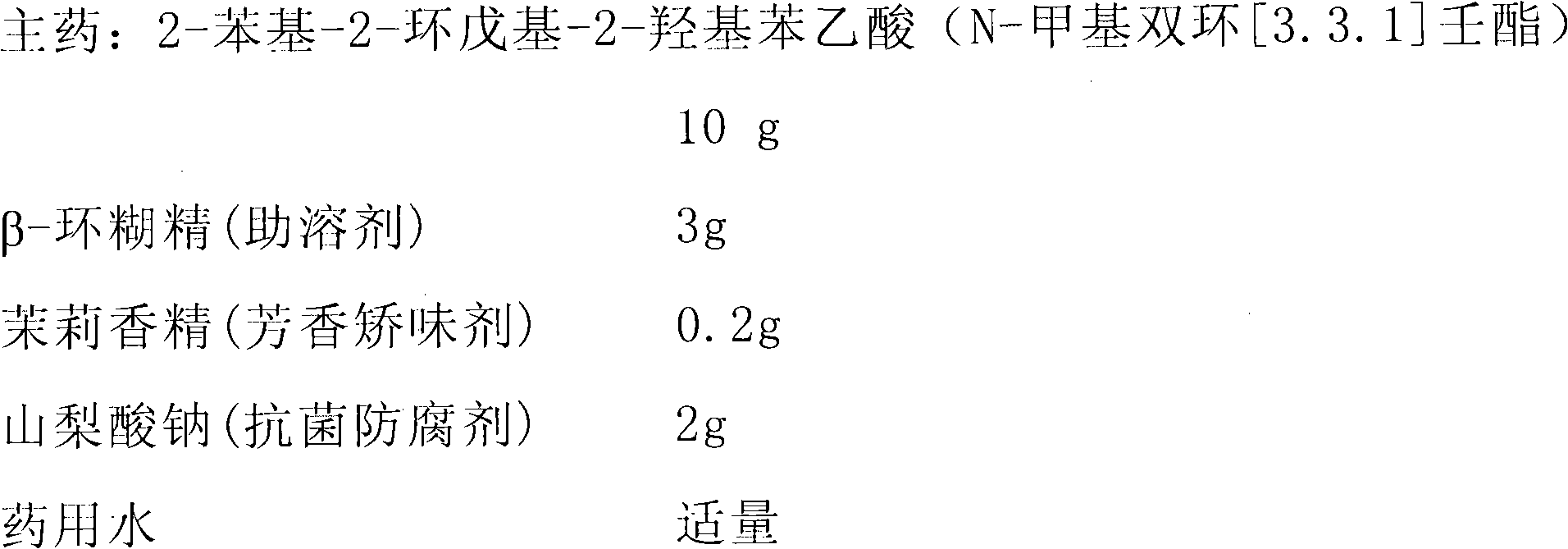
InactiveCN102836432AEasy to carryEasy to useOrganic active ingredientsNervous disorderNosePhenylacetic acid
The invention relates to a nasal preparation for treating epilepsy. The nasal preparation comprises a liquid preparation (nasal drops, nasal spray, collunarium), a nasal semisolid preparation (nasal smearing paste) and a nasal solid preparation (nasal powder). The nasal preparation comprises a main drug of an M receptor antagonist, such as the substituted hydroxyl phenylacetic acid (N-substituted bicyclic [3, 3, 1] nonane ester) and substituted hydroxyphenyl ethyl (N-substituted bicyclic [3, 3, 1] nonane ether). The nasal drug preparation is convenient to carry and use, and can be effectively treat and prevent seizure disorders.
Owner:伍丽娟
Nasal preparation for treating white-plus disease and preparation method thereof
ActiveCN101703754AEasy to administerLong durationOrganic active ingredientsHeavy metal active ingredientsCurative effectBULK ACTIVE INGREDIENT
The invention discloses a nasal preparation for treating white-plus disease and a preparation method thereof. The nasal preparation is prepared by taking a formula of the conventional Tibetan medicament white-plus unguent as a basic formula and modifying the formulation. In the invention, an adopted preparation method of extracting some medicinal materials and making other medicinal materials into superfine powder effectively retains the active ingredients of the medicinal materials, greatly improves the raw medicament component content and improves bioavailability so that the curative effect of the preparation is better. The auxiliary material selected and applied in the invention has good moldability so that a product has good permeability, better absorption and quicker response and the advantages of convenient administration, long duration, low allergy rate, convenient usage and carrying, no pollution to clothes and the like; and in the invention, a nasal mucosa administration system is adopted, so that the preparation has quick absorption, quick response, no stimulation nor unpleasant odor.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Nasal preparation for treating rhinitis and its preparation method and application
ActiveCN107412331BImprove complianceGood effectPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSide effectPatient compliance
The invention relates to the technical field of nasal preparations, and relates to a nasal preparation for treating rhinitis and its preparation method and application; the raw materials contain Xinjiang Artemisia chinensis, fried cocklebur, angelica dahurica, ephedra, scutellaria baicalensis extracts, Licorice extract, peppermint oil, Tween-80, excipients. The nasal preparation for treating rhinitis of the present invention is composed according to the theory of national medicine and traditional Chinese medicine. Compared with the prior art, the dosage of the nasal preparation for treating rhinitis of the present invention is only 20% of the dosage of the prior art. % to 30%, and the nasal preparation for the treatment of rhinitis of the present invention is more effective in anti-inflammation and anti-allergy than the prior art; meanwhile, the preparation process of the present invention is simple and convenient, has definite curative effect, no toxic and side effects, and the medicine directly acts on the nasal mucosa On the surface, it has high bioavailability, quick onset, convenience and good patient compliance. Different dosage forms can be prepared according to the needs of patients, which can be better used to treat acute, chronic and allergic rhinitis.
Owner:XINJIANG INST OF MATERIA MEDICA
A self-carrying carrier-free nasal cavity nano-preparation brain-targeted delivery system modified with chitosan oligosaccharides and its preparation method
ActiveCN109730966BReduce lossImprove compliancePowder deliveryNervous disorderPolythylene glycolOligosaccharide
The invention discloses a self-carrying carrier-free nasal cavity nano-preparation brain-targeted delivery system modified by chitosan oligosaccharides and a preparation method thereof. Including hydrophobic small molecule drugs with neuroprotective effects, polyethylene glycol derivatives, and chitosan oligosaccharides. The present invention also provides a preparation method for the brain-targeted delivery system of the nasal cavity nano-preparation. The first step is to prepare the freeze-dried powder of nanoparticles, and the second step is to stir the freeze-dried powder and chitosan oligosaccharide in isotonic saline before use to form a nasal cavity preparation with good membrane permeability. The preparation method of the system of the present invention is simple, can improve the hydrophobicity of small molecule drugs, reduce toxicity, and enhance neuroprotective effect; there is no carrier, no biodegradation problem and accumulation toxicity, the drug loading rate is as high as 25%, and the membrane is permeable after being modified by chitosan oligosaccharides Absorption is good, and the drug is delivered into the brain with high targeting. The administration method of the dosage form is nasal drop, spray, etc., and the operation is simple, which is convenient for patients who take medicine for a long time, and has a good application prospect in the treatment of nervous system diseases.
Owner:NASAL PHYTO SZ PHARMA TECH CO LTD
A medicament for treating cerebrovascular disease
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Pharmaceutical composition, preparation method and application of huperzine in-situ gel spray
InactiveCN1961879BIncrease brain selectivityEnhance memoryOrganic active ingredientsNervous disorderNasal cavityIrritation
The invention discloses a composition for constituting the in-situ gel spray for huperzine turbinitis, as well as its preparation method and application. The preparation is a nasal preparation which is in a liquid state in vitro and becomes a gel state when sprayed into a nasal cavity. It uses huperzine A as the active ingredient, supplemented with in-situ gel forming agent, isotonic regulator, pH regulator, preservative, water, etc. It is a nasal in-situ gel spray, which is suitable for the prevention and treatment of elderly people Dementia and middle-aged and elderly memory impairment or improve the memory and learning ability of adolescents. The nasal preparation is in a liquid state in vitro, the dosage is easy to accurately control, and it is convenient to use. When sprayed into the nasal cavity, it can be evenly dispersed on the surface of the nasal mucosa, and diffuses with the nasal mucus to form a gel, which stays in the nasal cavity for a long time and is not easy to be lost. The bioavailability of cerebrospinal fluid, brain, and hippocampus is 2.2 to 2.7 times that of oral administration, and the drug effect is 2 to 4 times that of oral administration. It has small local irritation, low toxicity, and good biocompatibility.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com