Novel coronavirus antibody with broad-spectrum neutralizing activity as well as preparation method and application of novel coronavirus antibody
A virus and antibody technology, applied in the field of new coronavirus antibodies and their preparation, can solve the problems of low antibody level, low antibody neutralization activity, and difficult to achieve, and achieve the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1, the preparation of immunogen
[0091] First, the preparation of recombinant plasmid
[0092] 1. Construction of wild-type RBD expression plasmid
[0093] The wild-type RBD expression plasmid was prepared according to the patent number "ZL 2010,801,084,7271", inventive Name as "a COVID-19 subunit vaccine and the preparation method thereof." Specific steps are as follows:
[0094] The double-stranded DNA molecules shown in the sequence table 2 were inserted into the NOTII and Xbai enzyme somewhere of the PCDNA3.1 vector (Saimi Fei Company, the item number V79020) to obtain recombinant plasmid pcDNA / NCOV-RBDFD. The recombinant plasmid has been sequenced.
[0095] The fusion protein shown in the DNA molecular coding sequence list shown in the sequence 2 in the sequence table, from the N-terminal to C end
[0096] The following functional components are included: human interleukin 10 signal peptide (1-18 in sequence 1) is used to secrete RBD recombinant proteins int...
Embodiment 2
[0123] Example 2, Preparation of anti-champional virus yolk antibody
[0124] First, immunization program
[0125] Experimental animals: 150 days old eggs (Tianjin Challenge Biotechnology Co., Ltd.).
[0126] Experimental method: The experimental animals were randomly divided into 8 groups for muscle injection, and 5 egg chickens per group were injected once every 3 weeks (3 times in total), and each group of treatment is specifically as follows:
[0127] Wild strain immunization group (RBD-WT): Injection of RBD-WT protein prepared in Example 1, 20 μg per injection dose, with white oil adjuvant (Zhejiang Zhengxin, Item No .: C-ZS-007), each injection 1 ml.
[0128] Mutant strain immunization group (RBD-MU): Injection of RBD-Mu protein prepared in Example 1, with an injection of 20 μg, with a white oil adjuvant, and 1 ml per injection.
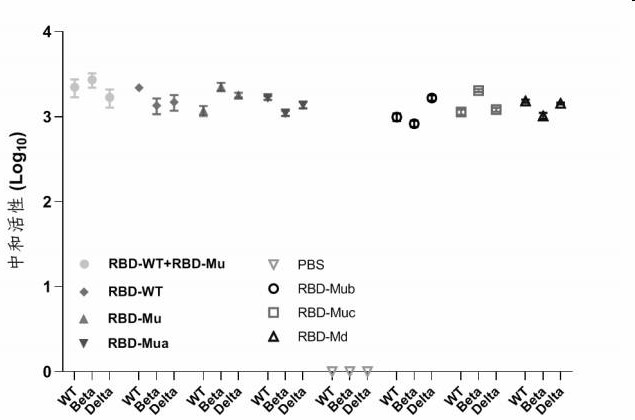
[0129] Hybrid immunochin group (RBD-WT + RBD-Mu): Injection of the RBD-WT protein and RBD-MU protein prepared in Example 1, each injection dose is...
Embodiment 3
[0143] Example 3, anti-champional virus oviviation antibody stability
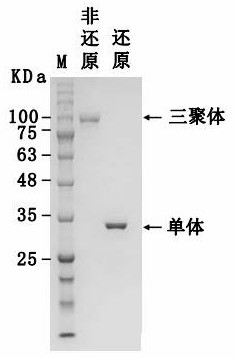
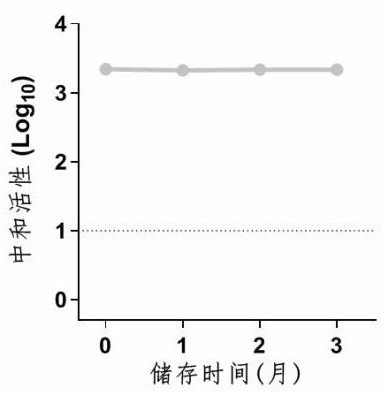
[0144] Example 2 was placed in an oval antibody solution (mixed immunity group) in 25 ° C for 3 months, and the activity was detected every other month, and SDS-PAGE electrophoresis was performed.
[0145] Such as image 3 and Figure 4 Indicated. The results show that antibodies have good thermal stability, and 25 ° C is placed in 3 months. These and active did not change, and the protein purity and concentration did not change significantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com