Novel edaravone preparation and preparation method thereof
A technology of edaravone and its preparation, which is applied in the new nasal administration dosage form of edaravone and its preparation field, which can solve the problems of less involvement in brain targeting, limited treatment of brain diseases, and low drug concentration.
Inactive Publication Date: 2016-08-24
NANJING HAIHENG MEDICINE SCI & TECH CO LTD
View PDF3 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0007] Since the 1990s, drug delivery systems, especially targeted drug delivery systems, have become a hot spot in pharmaceutical research, but current research is mainly focused on liver, spleen and lung targeting, and less involved in brain targeting.
The brain is an important part of targeted drug delivery. Many drugs must enter the brain to exert their curative effect. However, due to the existence of the blood-brain barrier, the drug concentration in the brain after conventional administration is relatively low, which limits the effect on the brain. treatment of internal diseases
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0031] The preparation of embodiment 1 nasal drops, spray
[0032]
[0033] According to the above prescription, add edaravone and auxiliary materials into an appropriate amount of water to make up to 1000mL, filter, sterilize, and pot, to obtain.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a preparation by which an edaravone drug can be targeted to a brain by virtue of a nasal delivery route, and a preparation method of the preparation. The edaravone nasal delivery preparation of the invention consists of an active ingredient, namely edaravone, and pharmaceutically acceptable effective adjuvant materials. The edaravone nasal preparation disclosed by the invention, after administration, can be rapidly absorbed and can rapidly reach the brain and enrich in the brain, so that a drug concentration in the brain, higher than that of an injection, which is administrated by an equivalent dosage, is provided, and a therapeutic effect on corresponding diseases is improved; meanwhile, by reducing edaravone concentrations in blood and other tissues, edaravone adverse reactions such as liver function damage and the like can be relieved; in addition, the compliance of patients can be improved; and the preparation is suitable for self-administration, so that the preparation, in the case of diseases, can be timely administrated as soon as possible.
Description
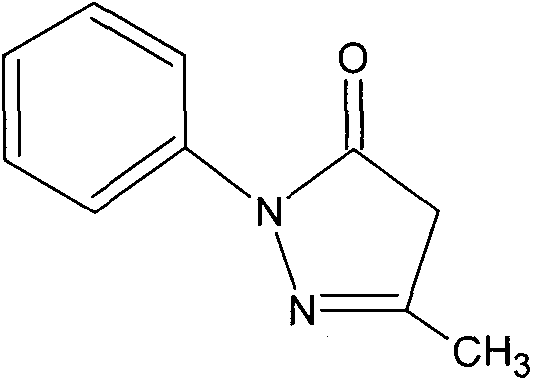
Technical field: [0001] The invention relates to a cranial nerve protective agent edaravone, in particular to a new nasal administration dosage form of edaravone and a preparation method thereof. Background technique: [0002] Edaravone is one of pyrazolone derivatives, its chemical name is 3-methyl-1-phenyl-2-pyrazolin-5-one, molecular formula C 10 h 10 N 2 O, the chemical structural formula is as follows: [0003] [0004] Edaravone was first developed by Japan's Mitsubishi Tokyo Pharmaceutical Company, and was approved for clinical use by the Ministry of Medical Labor and Welfare in Japan on April 4, 2001, with a trade name of Radicut. The drug is marketed in Japan in the form of water injection, the specification is 30mg / 20ml, the usual dosage is 30mg each time, twice a day, dissolved in normal saline or other diluents for intravenous infusion, generally used continuously for no more than 14 days, It is used to improve various mental and neurological symptoms and ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/00A61K31/4152A61P25/00A61P39/06A61P9/10
Inventor 钱世祥
Owner NANJING HAIHENG MEDICINE SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com