Pharmaceutical composition, preparation method and application of huperzine in-situ gel spray
A technology of huperzine A and in-situ gel, applied in the field of pharmacy, can solve the problems of toxic and side effects of the human body and low brain selectivity, and achieve the effects of less irritation, good compatibility and improving brain selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
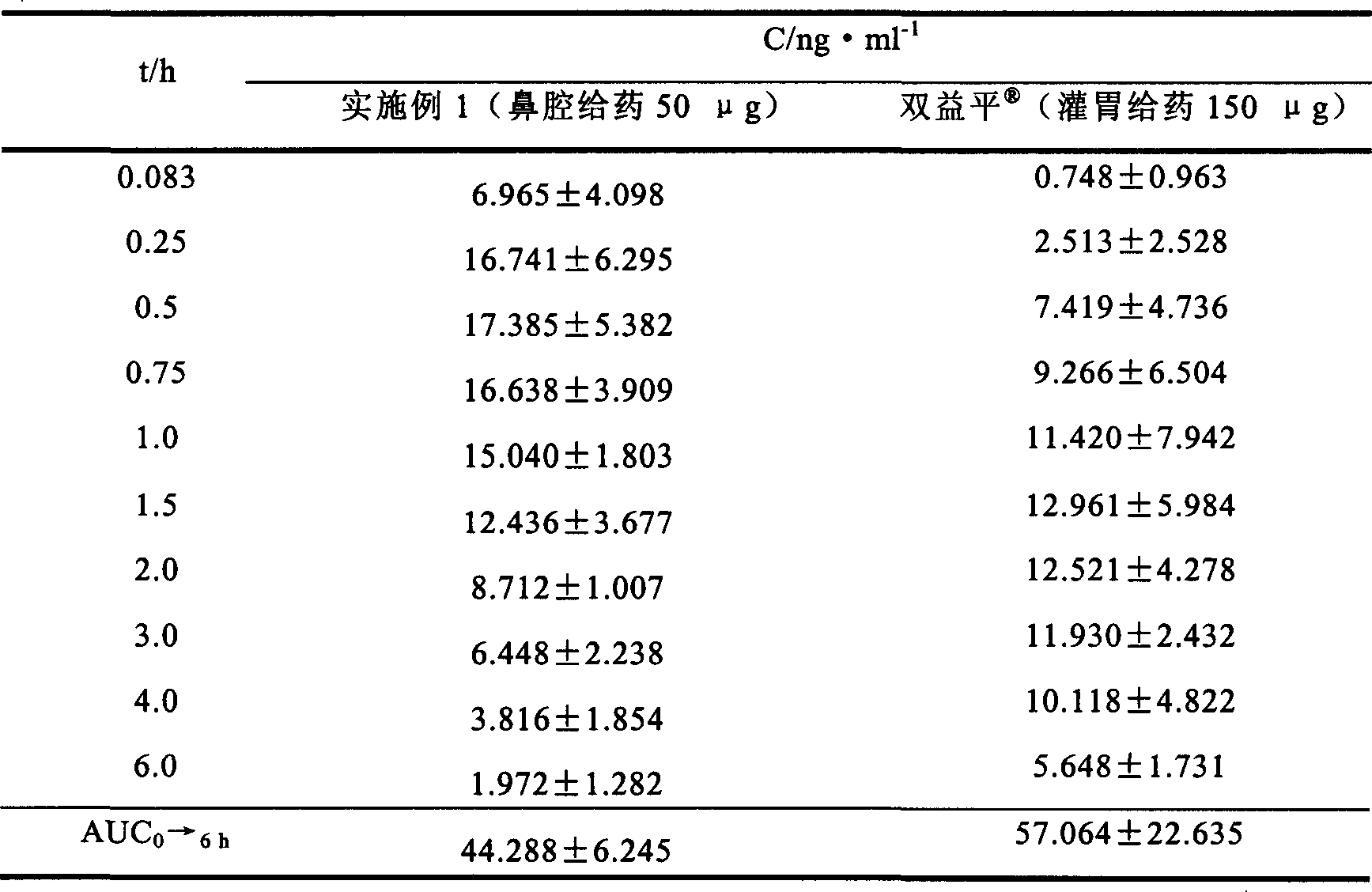
Embodiment 1
[0030] Preparation formula and preparation method:
[0031] Huperzine A 0.5g
[0032] Gellan Gum 5g
[0033] Mannitol 50g
[0034] Methylparaben 1.8g
[0035] Propylparaben 0.2g
[0036] Hydrochloric acid amount
[0037] Appropriate amount of ethanolamine
[0038] Add deionized water to 1000ml
[0039] Preparation method: Weigh the prescribed amount of mannitol, gellan gum, methylparaben and propylparaben, add 900ml of deionized water, and heat in a water bath at 90°C to dissolve completely. Dissolve Huperzine A with 30ml of 0.1mol / L hydrochloric acid, add it into the above solution under stirring, and mix well. Use 30% ethanolamine to adjust the pH to 6.5, add deionized water to reach the total amount, divide into white medical plastic bottles, and install a nasal cavity spray pump to obtain the product.
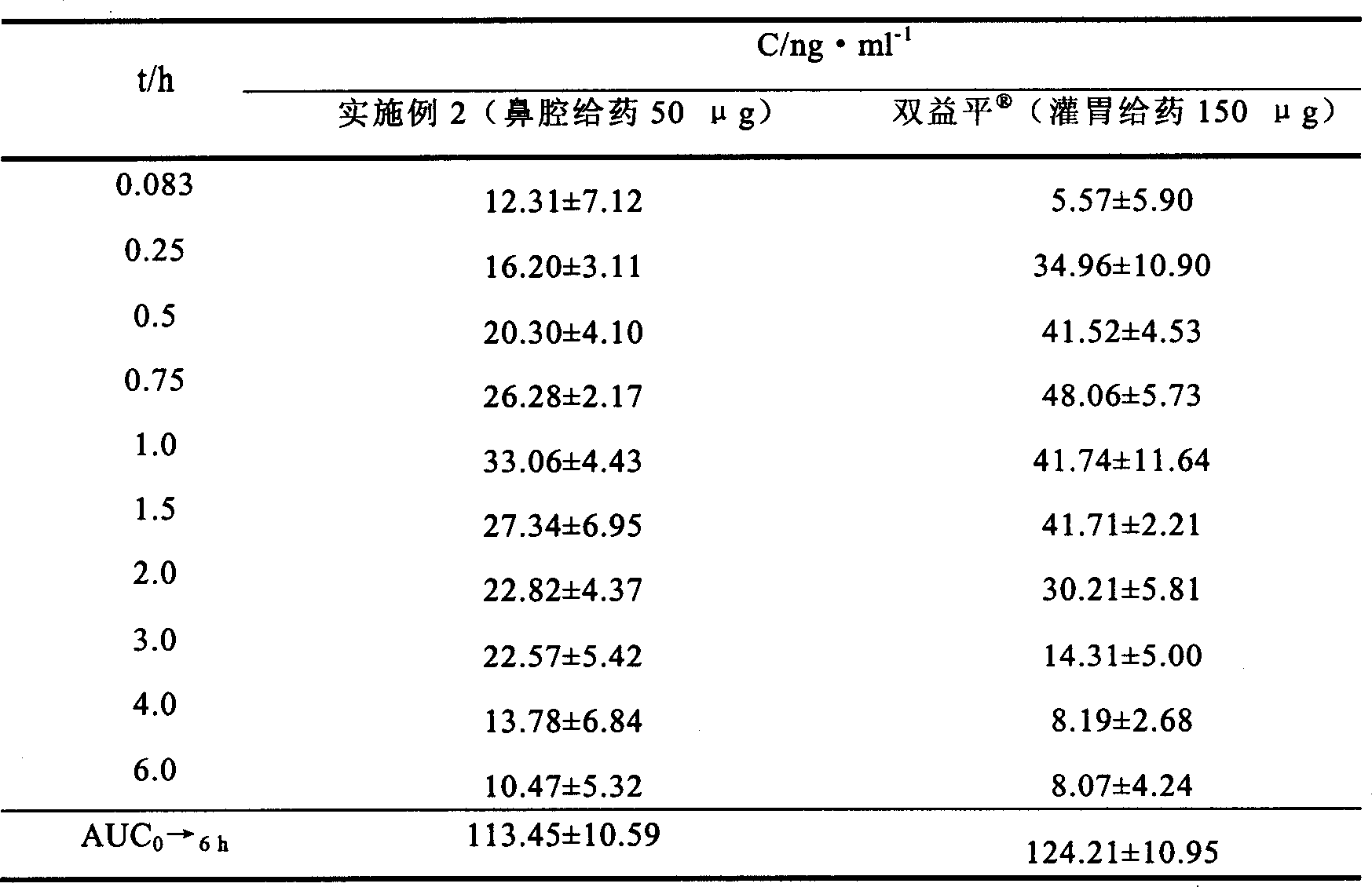
Embodiment 2
[0041] Preparation formula and preparation method:
[0042] Huperzine A 1g
[0043] Gellan Gum 5g
[0044] Mannitol 50g
[0045] Methylparaben 1.8g
[0046] Propylparaben 0.2g
[0047] Hydrochloric acid amount
[0048] Appropriate amount of trishydroxymethyl aminomethane
[0049] Add deionized water to 1000ml
[0050] Preparation method: Weigh the prescribed amount of mannitol, gellan gum, methylparaben and propylparaben, add 900ml of deionized water, and heat in a water bath at 90°C to dissolve completely. Dissolve huperzine A with 50ml of 0.1mol / L hydrochloric acid, add it into the above solution under stirring, and mix well. Use 30% tris to adjust the pH to 5, add deionized water to make the total amount, divide into white medical plastic bottles, and install a nasal cavity spray pump to obtain the product.
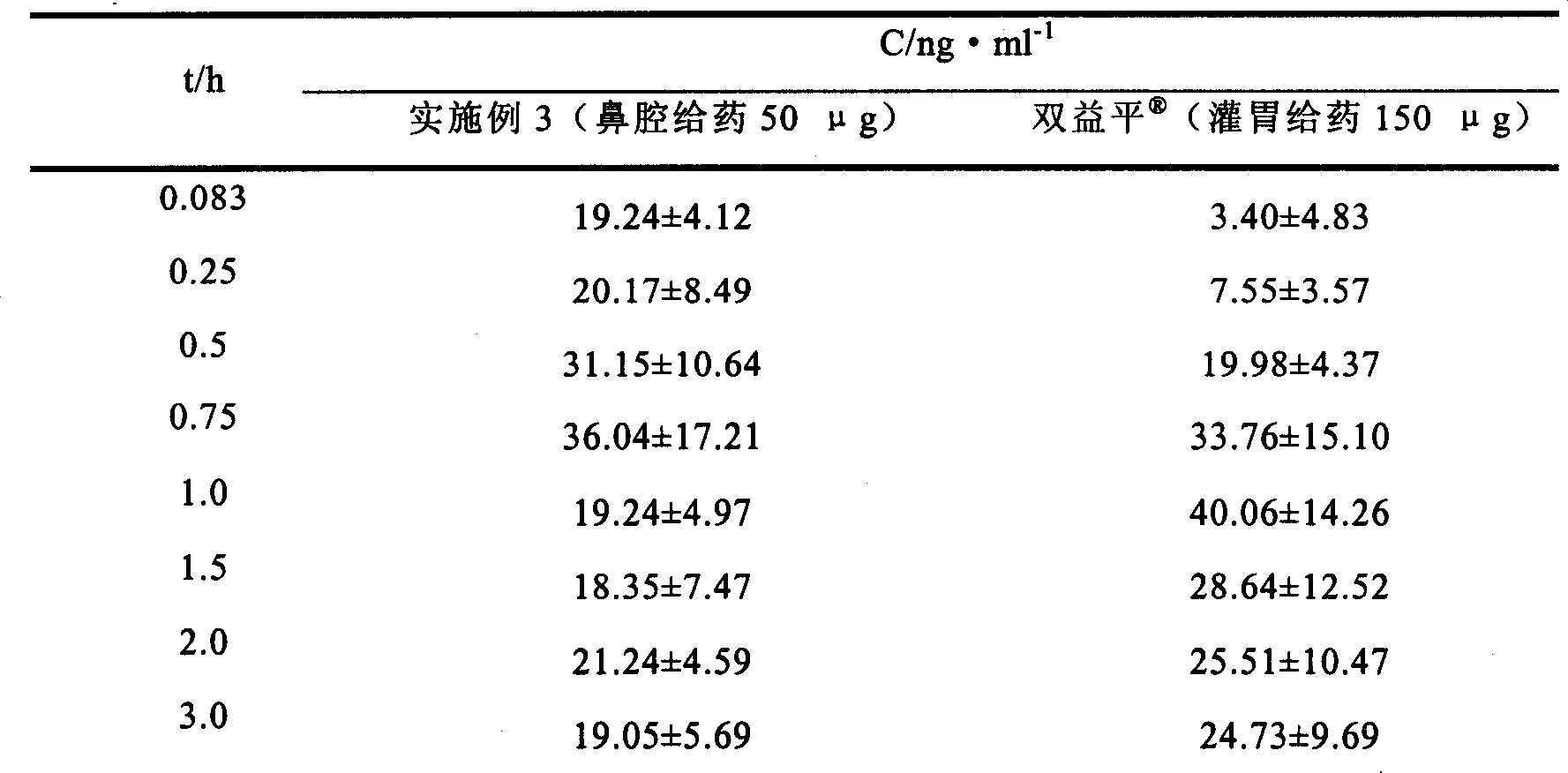
Embodiment 3
[0052] Preparation formula and preparation method:
[0053] Huperzine A 2g
[0054] Gellan Gum 6g
[0055] Glucose 50g
[0056] Methylparaben 1.8g
[0057] Propylparaben 0.2g
[0058] Hydrochloric acid amount
[0059] Appropriate amount of trishydroxymethyl aminomethane
[0060] Add deionized water to 1000ml
[0061] Preparation method: Weigh the prescribed amount of mannitol, gellan gum, methylparaben and propylparaben, add 850ml of deionized water, and heat in a water bath at 90°C to dissolve completely. Dissolve Huperzine A with 100ml of 0.1mol / L hydrochloric acid, add it into the above solution under stirring, and mix well. Use 30% tris to adjust the pH to 8, add deionized water to reach the total amount, divide into white medical plastic bottles, and install a nasal cavity spray pump to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com