Method for preparing flavonoid derivative containing pyrazole structural unit
A technology for structural units and flavonoids is applied in the field of preparation of flavonoid derivatives, and can solve the problems of harmful compound operation process, cumbersome, expensive reagents and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
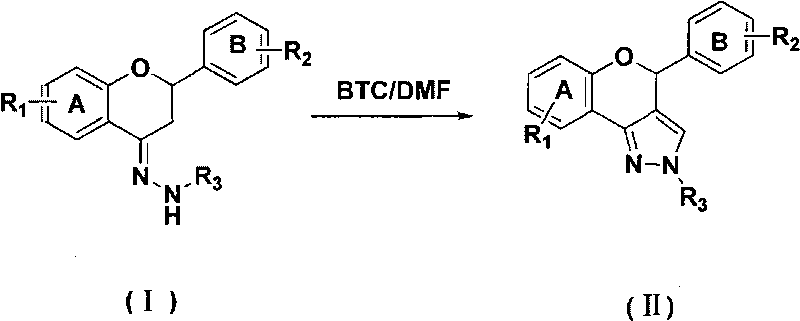
[0024] Embodiment 1: Preparation of flavonoid derivatives containing pyrazole structural unit
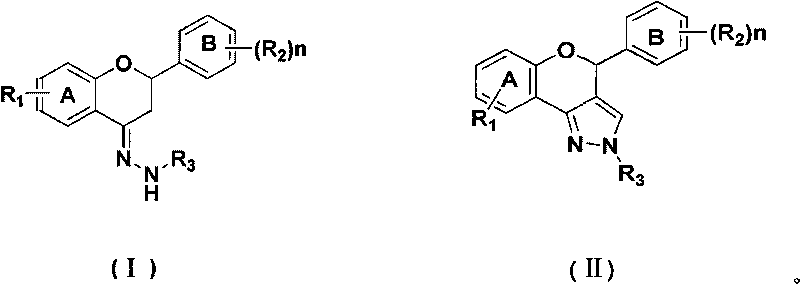
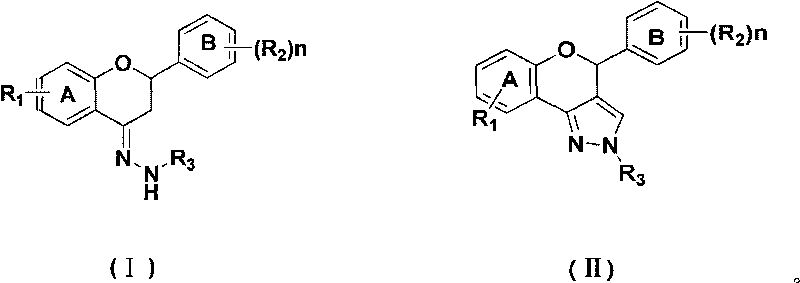
[0025] Product 2a: Put bis(trichloromethyl)carbonate (1.49g, 5mmol) into a dry 100mL three-neck flask equipped with mechanical stirring, drying tube, thermometer, etc., and cool to 0-5 At °C, a dichloromethane solution (4 mL) of N,N-dimethylformamide (1.31 g, 17.9 mmol) was added dropwise, the ice bath was removed after the drop, and the Vilsmeier reagent was prepared by allowing to rise to room temperature. Add the flavanone-4-hydrazone compound 1a (2.36g, 7.5mmol) represented by the prepared raw material formula (I), and heat the reaction mixture to 30-35°C for 4 hours. After the reaction, the reaction solution was added to ice water, stirred, and then saturated NaHCO 3 The pH of the solution was adjusted to neutral, and the layers were separated. The aqueous layer was extracted three times with dichloromethane (15ml×3). The dichloromethane layers were combined, washed with satur...
Embodiment 2
[0026] Embodiment 2: Preparation of flavonoid derivatives containing pyrazole structural unit
[0027] Product 2a: Put bis(trichloromethyl)carbonate (2.97g, 10mmol) into a 100mL three-necked flask equipped with mechanical stirring, drying tube, thermometer, etc., and cool to 0-5 At °C, a dichloromethane solution (8 mL) of N,N-dimethylformamide (2.63 g, 36 mmol) was added dropwise, the ice bath was removed after the drop, and the Vilsmeier reagent was prepared by allowing to rise to room temperature. Add the flavanone-4-hydrazone compound 1a (2.36g, 7.5mmol) represented by the prepared raw material formula (I), and heat the reaction mixture to 30-35°C for 4 hours. After the reaction, the reaction solution was added to ice water, stirred, and then saturated NaHCO 3The pH of the solution was adjusted to neutral, and the layers were separated. The aqueous layer was extracted three times with dichloromethane (15ml×3). The combined dichloromethane layers were washed with saturated ...
Embodiment 3
[0028] Embodiment 3: Preparation of flavonoid derivatives containing pyrazole structural unit
[0029] Product 2a: Put bis(trichloromethyl)carbonate (4.46g, 15mmol) into a dry 100mL three-neck flask equipped with mechanical stirring, drying tube, thermometer, etc., and cool to 0-5 At °C, a dichloromethane solution (12 mL) of N,N-dimethylformamide (3.94 g, 54.0 mmol) was added dropwise, the ice bath was removed after the drop, and the Vilsmeier reagent was prepared by allowing to rise to room temperature. Add the flavanone-4-hydrazone compound 1a (2.36g, 7.5mmol) represented by the prepared raw material formula (I), and heat the reaction mixture to 30-35°C for 4 hours. After the reaction, the reaction solution was added to ice water, stirred, and then saturated NaHCO 3 The pH of the solution was adjusted to neutral, and the layers were separated. The aqueous layer was extracted three times with dichloromethane (15ml×3). The combined dichloromethane layers were washed with satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com