Technology for removing hydrogen sulfide in gas at room temperature
一种脱除气体、硫化氢的技术,应用在气体处理、硫的制备/提纯、化学仪器和方法等方向,能够解决不适合湿法循环脱硫、羟基氧化铁含量高、吸收剂消耗大等问题,达到克服物料消耗大、脱硫速度快、再生速度和效率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
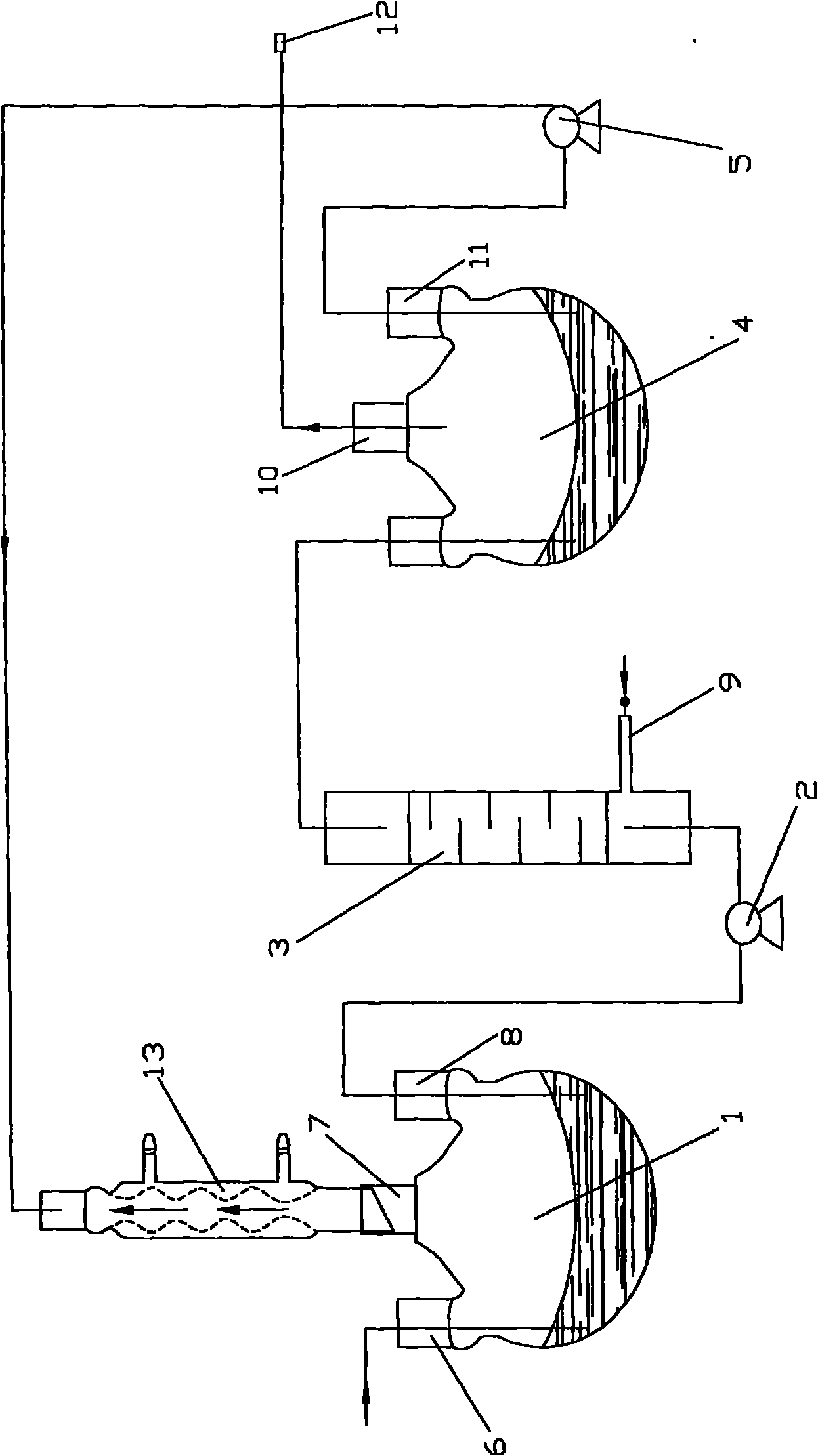
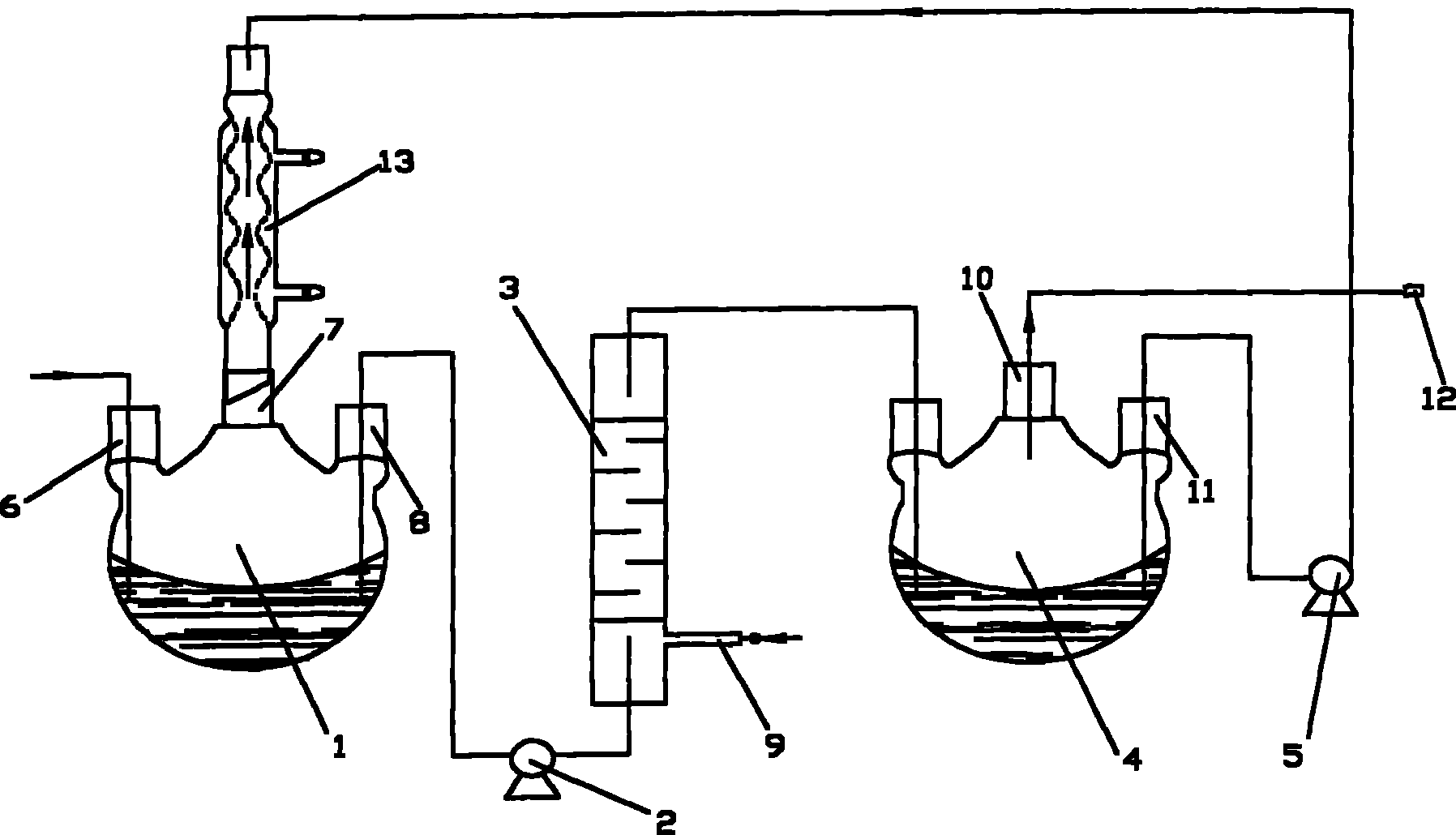
Image
Examples
Embodiment 1
[0032] Preparation of materials containing amorphous ferric oxyhydroxide:
[0033] 456 g FeSO 4 ·7H 2O is made into an aqueous solution and placed in a reaction kettle, and the aqueous solution made of 135 grams of solid NaOH is added under stirring conditions, and the reaction temperature does not exceed 70°C by controlling the feeding rate of the NaOH solution. After the reaction is completed, filter and wash the filter cake with water. , until the Na in the filter cake + The content of the filter cake is less than 0.5%, and then the filter cake is made into a water suspension with a solid mass percentage of 30%, and the air is passed through for oxidation until Fe 2+ / Fe 总 If it is less than 1%, the material is completely oxidized, filtered, and dried at 90°C to obtain a material containing amorphous iron oxyhydroxide, wherein the mass percentage of amorphous iron oxyhydroxide is 85%, and the rest is Na 2 SO 4 , water and TiO 2 (TiO 2 for industrial FeSO 4 ·7H 2 O)...
Embodiment 2
[0037] Preparation of Materials Containing Amorphous Iron Oxyhydroxide:
[0038] Fe(NO 3 ) 2 ·6H 2 O is made into an aqueous solution and placed in a reaction kettle, and the aqueous solution made of solid NaOH is added under stirring conditions, and the reaction temperature is maintained at 30-40°C by controlling the feeding speed of the NaOH solution, and the pH of the solution at the end of the reaction is controlled = 7.5 , filter the solution, and wash the filter cake with water until Na in the filter cake + The content of the filter cake is less than 0.5%, and then the filter cake is made into a water suspension with a solid mass percentage of 10%, and the air is passed through for oxidation until Fe 2+ / Fe 总 If it is less than 1%, the material is completely oxidized, filtered, and dried at 70°C to obtain a material containing amorphous iron oxyhydroxide, wherein the mass percentage of amorphous iron oxyhydroxide is 100%. The sulfur capacity of the amorphous iron ox...
Embodiment 3
[0043] Preparation of Materials Containing Amorphous Iron Oxyhydroxide:
[0044] Make KOH into an aqueous solution and place it in a reaction kettle, and add solid FeCl under stirring conditions 2 formulated aqueous solution, by controlling the FeCl 2 The feed rate of the solution keeps the reaction temperature at 40-50° C., and controls the pH of the solution at the end of the reaction=8, filters the solution, and washes the filter cake with water until K in the filter cake. + The content of the filter cake is less than 0.5%, and then the filter cake is made into a water suspension with a solid mass percentage of 15%, and the air is passed through for oxidation until Fe 2+ / Fe 总 If it is less than 1%, the material is completely oxidized, filtered, and dried at 60°C to obtain a material containing amorphous iron oxyhydroxide. The mass percentage of amorphous iron oxyhydroxide in the material is 92%, and the remaining components are KCl , water and unknown impurities, the su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com