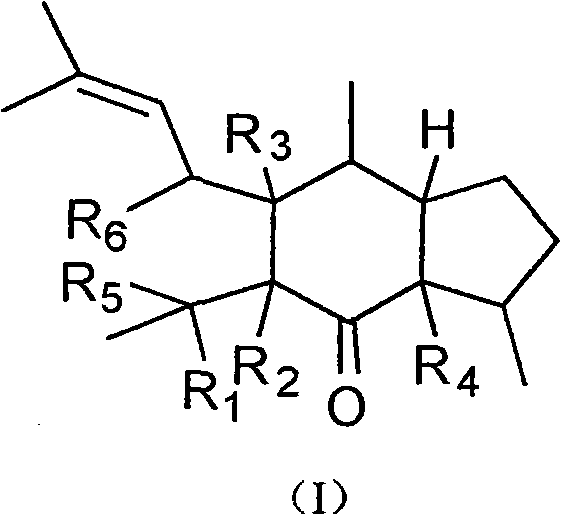
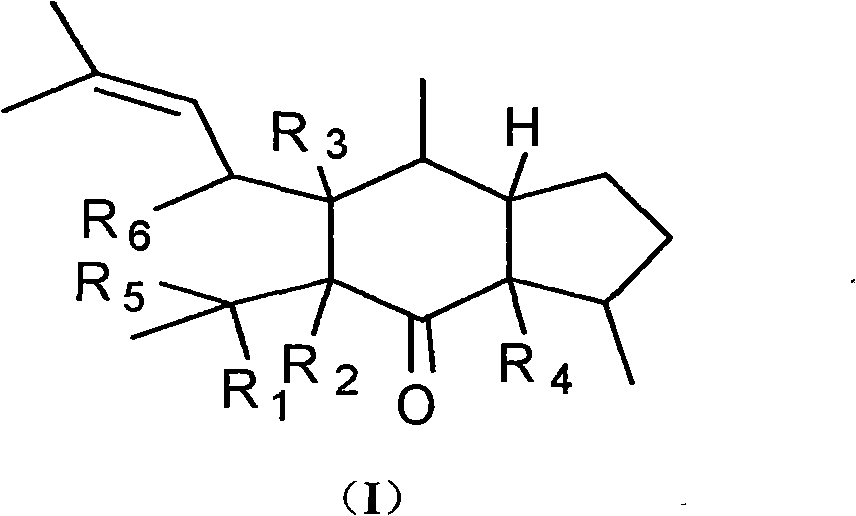
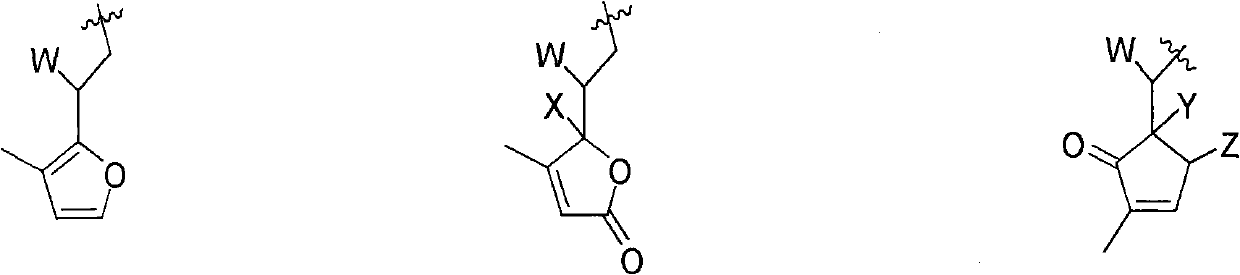
Dimeric sesquiterpene compound and applications thereof
A compound and group technology, applied in the field of natural medicinal chemistry, to achieve unique aroma characteristics, good hair permeability, and good resistance to prolyl endopeptidase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Extraction and separation of compound 1:
[0045] 1. Extraction and separation:
[0046] Leaves of Leucosceptrum canum Smith (12.5kg) were dried in the shade, crushed to 30 mesh, extracted by cold immersion in n-hexane for 3 times (each time 40 liters, 24 hours), the extracts were combined, and the extracts were concentrated under reduced pressure Obtain medicinal extract (203g), the medicinal extract is mixed with silica gel (200-300 mesh) sample after dissolving appropriate amount of chloroform / acetone, 1.5kg silica gel (200-300 mesh) carries out coarse separation with thick and short type silica gel column, with different proportions Petroleum ether-chloroform (1:0-0:1), chloroform-acetone (1:0-0:1) for gradient elution, each 1000mL as a fraction, a total of 400 parts. TLC detection merged the same fractions to obtain 9 main fractions. Among them, the sixth part (40g) uses MCI-gel (60-100% MeOH-H 2 (0) After treatment, 4 parts were obtained, and then Fr6a-3 (85% M...
Embodiment 2
[0050] Extraction, separation and structure identification of compound 2:
[0051] 1. Extraction and separation:
[0052] According to Example 1, the flowers and leaves of rice balls are extracted and separated, and then Fr6a-4 (89% MeOH, 10g) is subjected to silica gel column chromatography with petroleum ether: ethyl acetate (35: 1) as the eluting solvent, and every 100mL As a fraction, 150 parts were collected, of which 40-96 parts Sephadx-LH20 (CHCl 3 : MeOH = 1: 1) and then semi-preparative liquid phase (80% MeOH: H 2 O) Purification afforded compound 2 (300 mg).
[0053] 2. Compound structure identification:
[0054] The compound is a colorless oil; optical activity, [α] D 29 =+55.5 (c=0.9, solvent is chloroform); UV spectrum (solvent is chloroform), λ max (logε)=240(3.06)nm; infrared spectrum (potassium bromide tablet)ν max =3442, 2962, 2934, 2870, 1702, 1452, 1376, 1276, 1150, 1052, 983, 727cm -1 ;negative FAB-MS m / z(%): 399(20)[M-H] - , 381(59), 279(100), 255...
Embodiment -3
[0056] Extraction, separation and structure identification of compound 3:
[0057] 1. Extraction and separation
[0058] Leucosceptrum canum Smith leaves (5.2kg) were dried in the shade, crushed to 30 mesh, extracted 3 times with methanol hot reflux (15 liters each time, 4 hours), the extracts were combined, and the extracts were concentrated under reduced pressure to obtain Total extract (125g), total extract is extracted with ethyl acetate (4 times, each 3 liters) to obtain ethyl acetate extract (65g), ethyl acetate extract is dissolved with silica gel (200 -300 mesh) mixed sample, 0.5kg silica gel (200-300 mesh) was roughly separated with a thick and short silica gel column, with different ratios of petroleum ether-chloroform (1:0-0:1), chloroform-acetone (1: 0-0:1) gradient elution, each 500mL as a fraction, a total of 300 parts. TLC detection merged the same fractions to obtain 8 main fractions. Wherein the 6th part (28g) uses MCI-gel (60-100% MeOH-H 2 (0) After treat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com