Recombinant lentivirus for carrying out targeted induction on osteogenic differentiated cell apoptosis as well as preparation method and application thereof
A technology of osteogenic differentiation cells and recombinant lentivirus, applied in biochemical equipment and methods, methods based on microorganisms, bone diseases, etc., can solve the problems of limited repair ability of cartilage, lack of treatment measures, etc., and achieve low cost and high preparation method easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] Hereinafter, preferred embodiments of the present invention will be described in detail with reference to the accompanying drawings. The experimental method that does not indicate specific conditions in the preferred embodiment is usually according to conventional conditions, such as described in the Molecular Cloning Experiment Guide (Third Edition, J. Sambrook et al., translated by Huang Peitang, etc., Science Press, 2002) conditions, or as recommended by the manufacturer. Unless otherwise stated, various restriction endonucleases, DNA polymerases, DNA ligases and kits in the preferred examples were purchased from Shanghai Sangon Bioengineering Technology Service Co., Ltd. and Beijing Biotech Biogene Technology Co., Ltd. company.
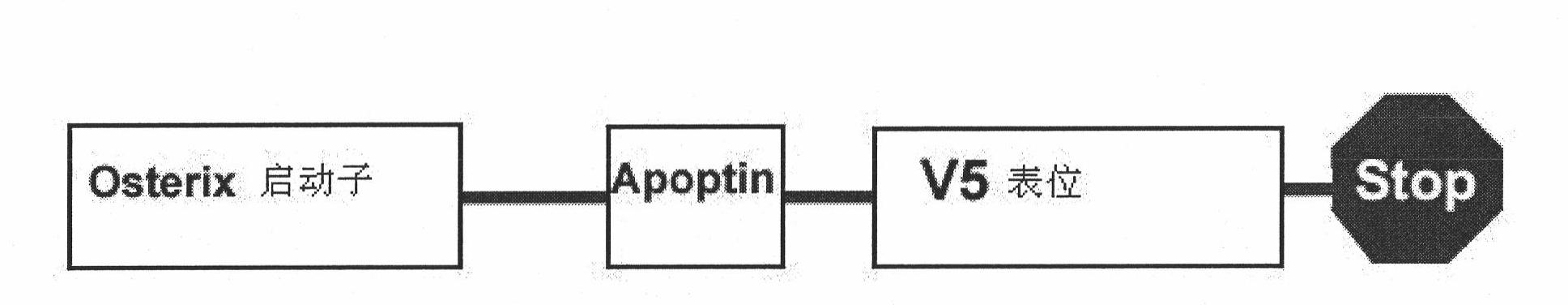
[0036] 1. Preparation of Osxp-Apoptin recombinant lentivirus
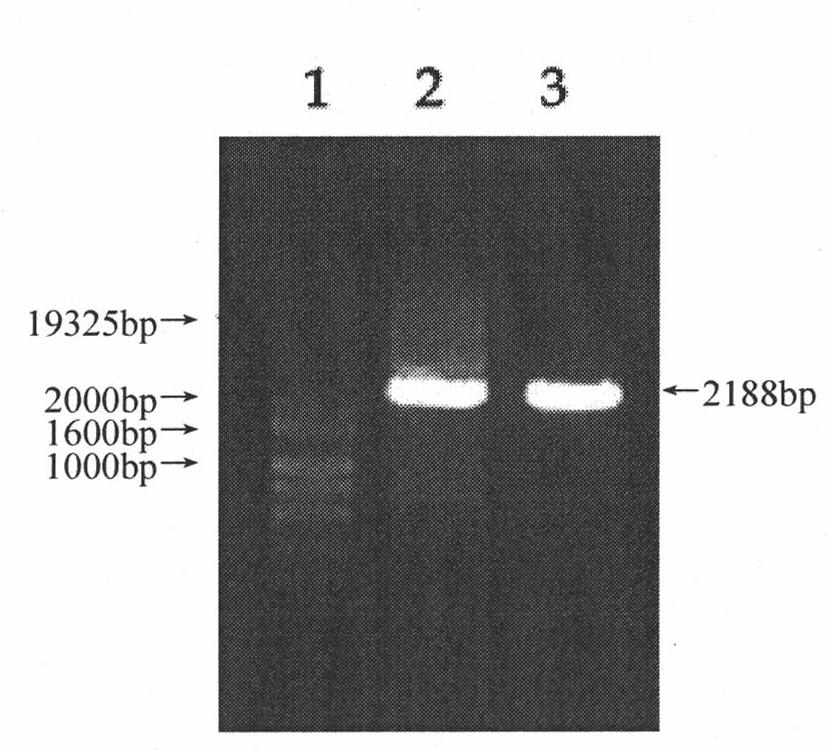
[0037] The schematic diagram of the construction of Osxp-Apoptin recombinant lentivirus is as follows figure 1 Shown, its preparation method comprises the following steps:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com