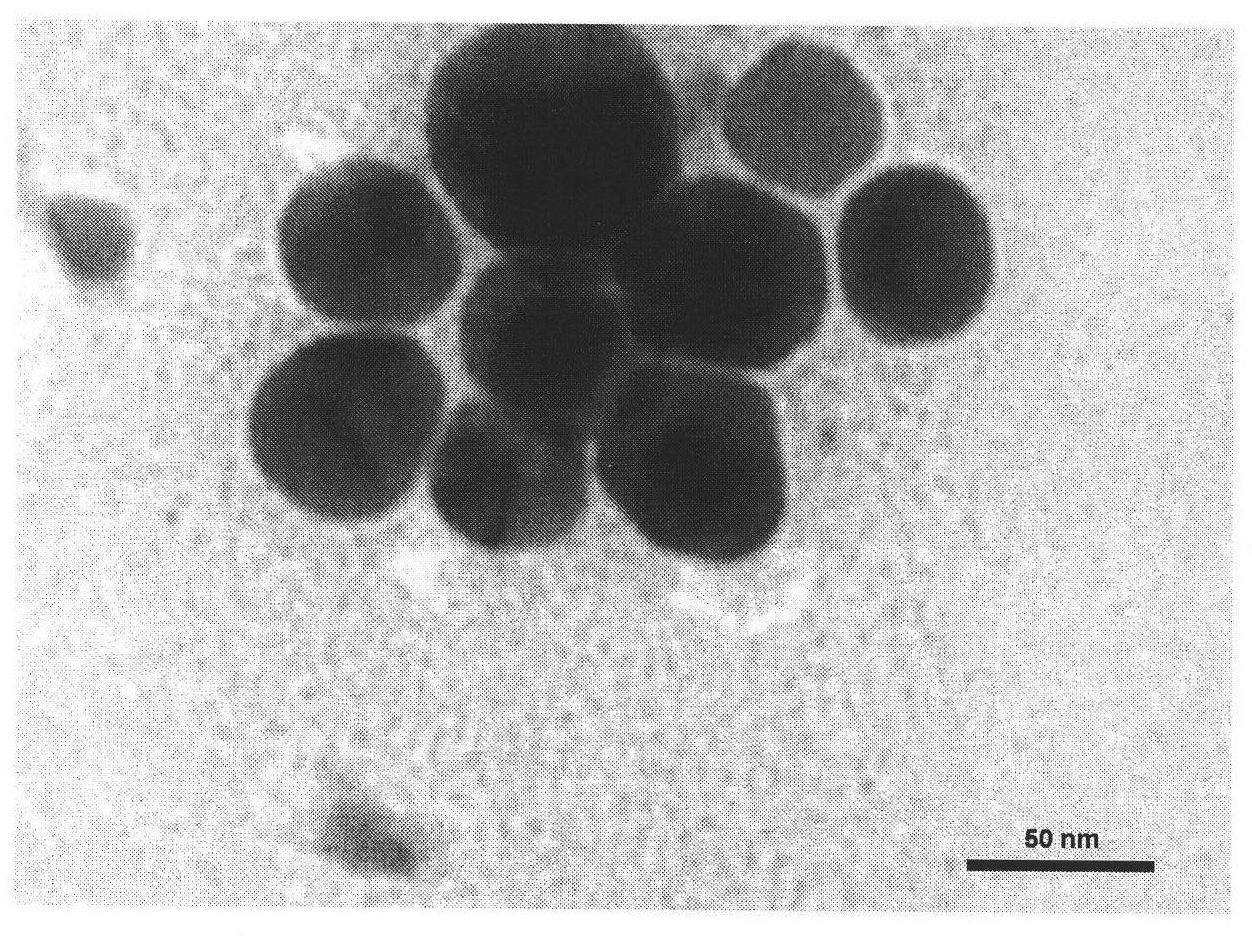
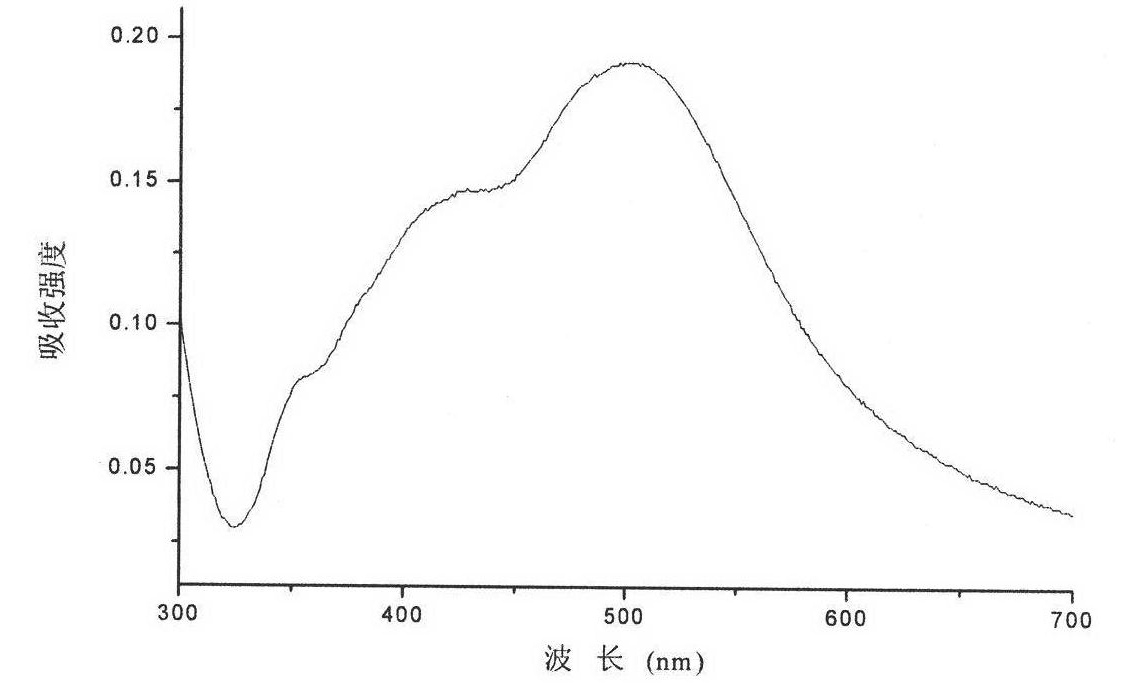
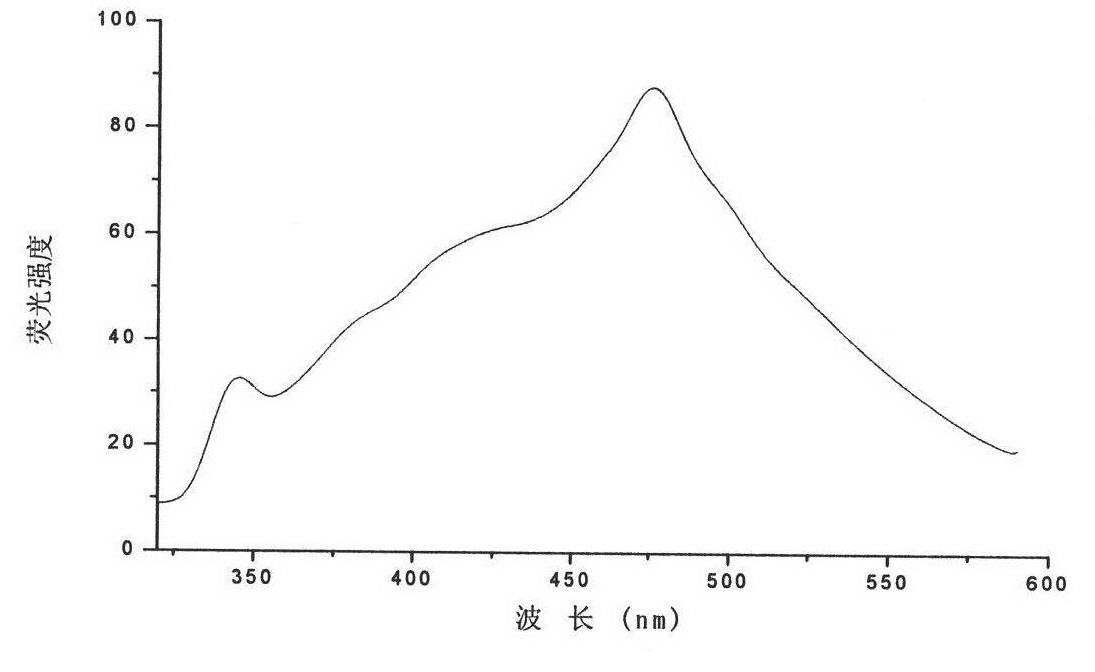
Fluorescent silver nanoparticle and preparation method thereof
A technology of silver nanoparticles and fluorescence, which is applied in the direction of chemical instruments and methods, luminescent materials, etc., can solve problems that have not been reported, and achieve the effect of simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At 70°C, 3 μmol AgNO 3 Add it into 10 mL of pure water containing 5 mM cetyltrimethylammonium chloride and 2 mM ascorbic acid, and stir for 2 h in the dark to obtain fluorescent silver nanoparticles. The fluorescence emission peak of the silver nanoparticles was measured at 475nm on a fluorescence spectrophotometer. Under natural light, the silver nanoparticles fluoresced blue-green when viewed against a black background. Its fluorescence quantum yield is 0.5%.
Embodiment 2
[0021] At 100°C, 10 μmol AgNO 3 Add it into 10 mL of pure water containing 0.1 mM cetyltrimethylammonium bromide and 2 mM ascorbic acid, and stir for 15 minutes in the dark to react to obtain fluorescent silver nanoparticles. The fluorescence emission peak of the silver nanoparticle is at 440nm, and when observed with a black background under natural light, the silver nanoparticle emits blue fluorescence. Its fluorescence quantum yield is 0.7%.
Embodiment 3
[0023] At 90°C, 3 μmol AgNO 3 Add it into 10 mL of pure water containing 5 mM cetyltrimethylammonium chloride and 2 mM ascorbic acid, and let it stand in the dark for 1 h to obtain fluorescent silver nanoparticles. The fluorescent emission peak of the silver nanoparticle is a broad peak between 425nm and 475nm, and when observed with a black background under natural light, the silver nanoparticle emits blue fluorescence. Its fluorescence quantum yield is 0.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com