Method for preparing highly ordered tungsten trioxide nano-rod
A tungsten trioxide and nanorod technology, applied in tungsten oxide/tungsten hydroxide and other directions, can solve the problems of difficult growth, high cost, unfavorable tungsten trioxide nanorod material, etc., and achieve good repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Mix 25 milliliters of deionized water and 25 milliliters of commercially available concentrated hydrochloric acid with a weight percentage of 36.5% to achieve a mixed solution with a total volume of 50 milliliters; after stirring at room temperature for 5 minutes, add 0.2 grams of tungsten hexachloride; continue After stirring for 5 minutes, transfer to a 100 ml high-temperature reaction kettle (lined with tetrafluoroethylene); put the high-temperature reaction kettle in a constant temperature drying oven, and cool it naturally after hydrothermal reaction at a constant temperature of 120°C for 10 hours; Take it out from the high-temperature reaction kettle, wash it with deionized water, and then dry it in a drying oven. The obtained nanorods have a diameter of 80-90 nanometers and a length of 3-4 microns.
Embodiment 2
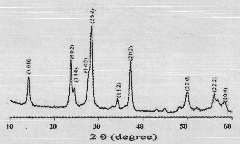
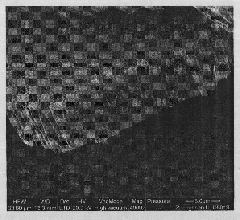
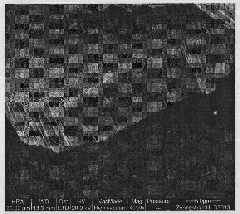
[0013] Mix 25 milliliters of deionized water and 25 milliliters of commercially available concentrated hydrochloric acid with a weight percentage of 38% to achieve a mixed solution with a total volume of 50 milliliters; after stirring at room temperature for 5 minutes, add 0.39 grams of tungsten hexachloride; continue After stirring for 5 minutes, transfer to a 100 ml high-temperature reaction kettle (lined with tetrafluoroethylene); place the high-temperature reaction kettle in a constant temperature drying oven, and cool it naturally after hydrothermal reaction at a constant temperature of 150°C for 20 hours; Take it out from the high-temperature reaction kettle, wash it with deionized water, and then dry it in a drying oven. The obtained nanorods have a diameter of 90-100 nanometers and a length of 5-6 microns. The XRD pattern of the prepared tungsten trioxide nanorods is shown in figure 1 , the SEM image of the side of the prepared tungsten trioxide nanorods is shown in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com