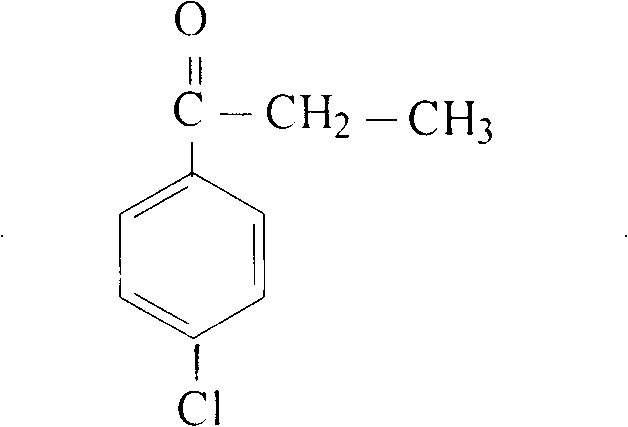
Preparation method of p-chloropropiophenone
A technology of chloropropiophenone and p-chlorobenzoic acid, which is applied in the field of pharmaceutical intermediates, can solve problems such as high product cost, unsatisfactory results, and complicated operation process, and achieve low cost, moderate reaction time, and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a four-necked reaction flask equipped with an electric stirrer, a reflux condenser, and a thermometer, add 15.65 g of p-chlorobenzoic acid, 51.8 g of propionic acid, and 2.5 g of catalyst in sequence. After adding the materials, start stirring and control the condensation reaction temperature to 140°C. For about 12 hours, carry out the condensation reaction by stirring for 12 hours, distill off excess propionic acid, and continue to rise in temperature until carbon dioxide gas begins to escape at 280°C, and carry out decarboxylation reaction at a temperature of 280°C-285°C, while using 95% ethanol solution Absorb the escaped carbon dioxide gas and distilled liquid, stop the reaction when there is no liquid distilled out in the four-port reactor after decarboxylation reaction for 2 hours, then cool and crystallize to obtain p-chloropropiophenone product, the yield of p-chloropropiophenone It reaches 86.45%, and the melting point is 34-37°C.
Embodiment 2
[0020] In a four-necked reaction flask equipped with an electric stirrer, a reflux condenser, and a thermometer, add 15.65 g of p-chlorobenzoic acid, 51.8 g of propionic acid, and 3.5 g of catalyst in sequence. After adding the materials, start stirring and control the condensation reaction temperature to 140°C. For about 12 hours, carry out the condensation reaction by stirring for 12 hours, distill off excess propionic acid, and continue to rise in temperature until carbon dioxide gas begins to escape at 280°C, and carry out decarboxylation reaction at a temperature of 280°C-285°C, while using 95% ethanol solution Absorb the escaped carbon dioxide gas and the distilled liquid, stop the reaction after 2 hours when there is no liquid distilled out in the four-port reactor, then cool and crystallize to obtain the p-chloropropiophenone product, and the yield of p-chloropropiophenone reaches 87.71 %, the melting point is 34-37°C.
Embodiment 3
[0022] In a four-necked reaction flask equipped with an electric stirrer, a reflux condenser, and a thermometer, add 15.65 g of p-chlorobenzoic acid, 59.2 g of propionic acid, and 2.5 g of catalyst in sequence. After adding the materials, start stirring and control the condensation reaction temperature to 140°C. For about 12 hours, carry out the condensation reaction by stirring for 12 hours, distill off excess propionic acid, and continue to rise in temperature until carbon dioxide gas begins to escape at 280°C, and carry out decarboxylation reaction at a temperature of 280°C-285°C, while using 95% ethanol solution Absorb the escaped carbon dioxide gas and the distilled liquid, stop the reaction after 2 hours when there is no liquid distilled out in the four-port reactor, then cool and crystallize to obtain the p-chloropropiophenone product, and the yield of p-chloropropiophenone reaches 86.81% %, the melting point is 34-37°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com