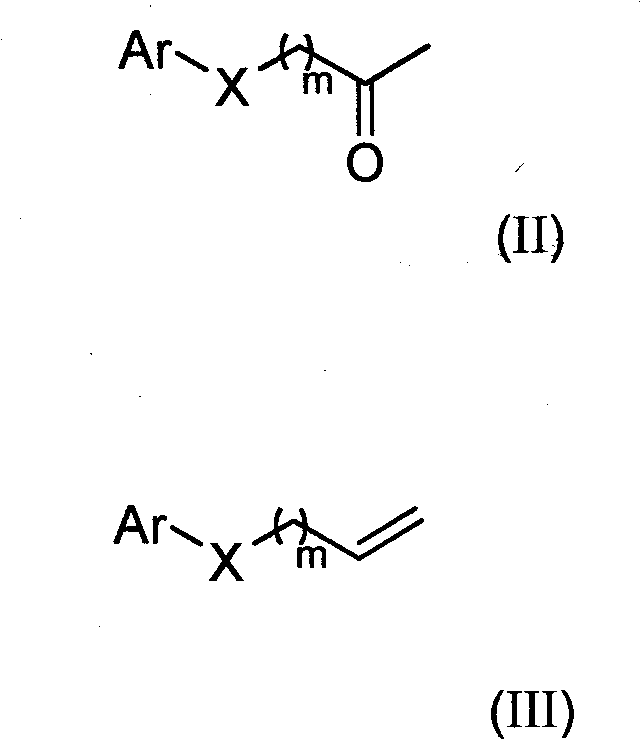
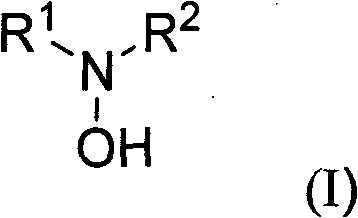
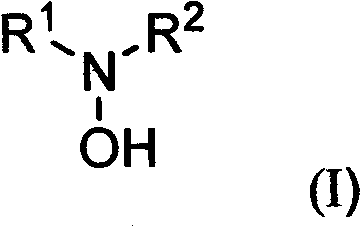Substituted hydroxylamine derivatives with anti-inflammatory activity and preparation method and application thereof
A technology of substituents and representatives, applied in the field of substituted hydroxylamine derivatives with anti-inflammatory activity, their preparation and application, and able to solve problems such as application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 1-Phenyl-3-thiosemicarbazide
[0071] Phenylhydrazine hydrochloride (6.0g, 0.042mol) was suspended in 40ml of absolute ethanol, 0.5ml of concentrated hydrochloric acid was added dropwise, the temperature was raised to about 40°C, KSCN (4.9g, 0.050mol) was added in batches for 40min, and the temperature was raised to reflux for 6h. After suction filtration, the filtrate was allowed to stand still, and a large amount of precipitate was precipitated, and then suction filtration gave 4.5 g of white powder, yield: 65.2%, m.p.197-200°C.
Embodiment 2
[0073] p-methylsulfonylbenzoyl chloride
[0074] p-Methanesulfonylbenzoic acid (10 g, 0.05 mol) was suspended in SOCl 2 (25ml, 0.21mol), heated and refluxed for 2.5h, concentrated to dryness under reduced pressure to obtain a white yellowish solid, which was recrystallized from toluene to obtain 8.5g of white solid, yield: 78%, m.p.128-130°C.
Embodiment 3
[0076] 2-(4-Methanesulfonylbenzoyl)-2-phenyl-thiosemicarbazide
[0077] Suspend 1-phenyl-3-thiosemicarbazide (0.6g, 3.6mmol) in 20ml of anhydrous acetone, add triethylamine 5d, stir at room temperature, add p-methylsulfonylbenzoyl chloride (0.79g , 3.6mmol), heated to reflux for 3h, suction filtered, and the filter cake was dried to obtain 1.1g of white solid, yield: 87.8%, m.p.204-206°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com