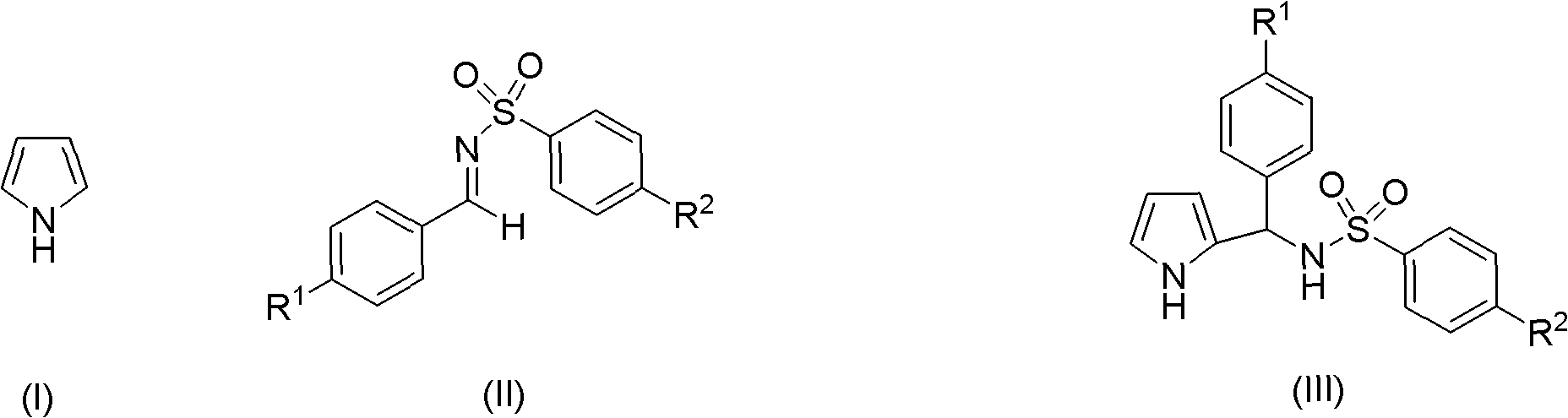
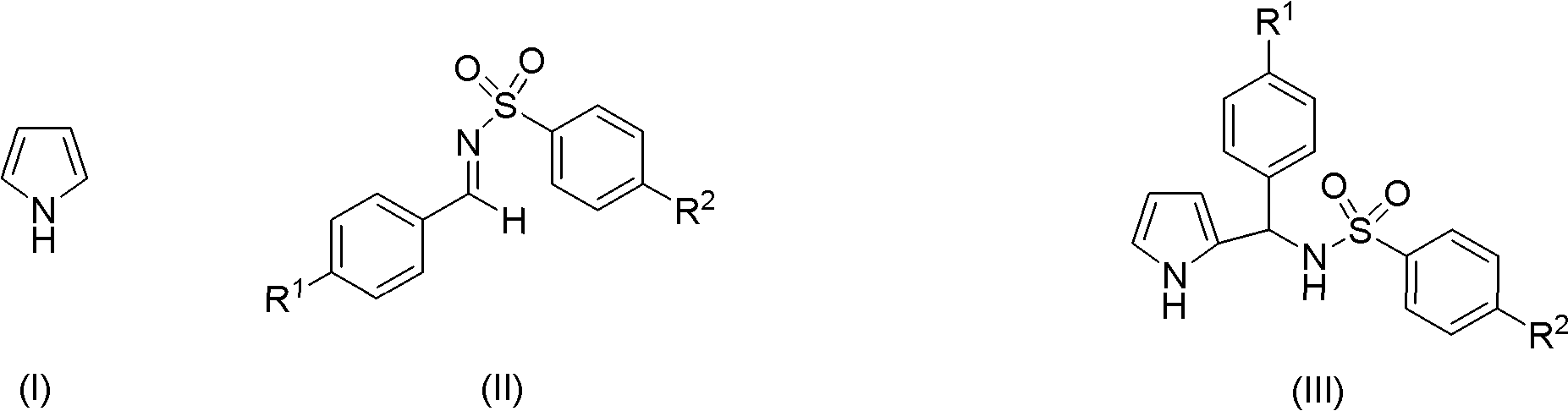
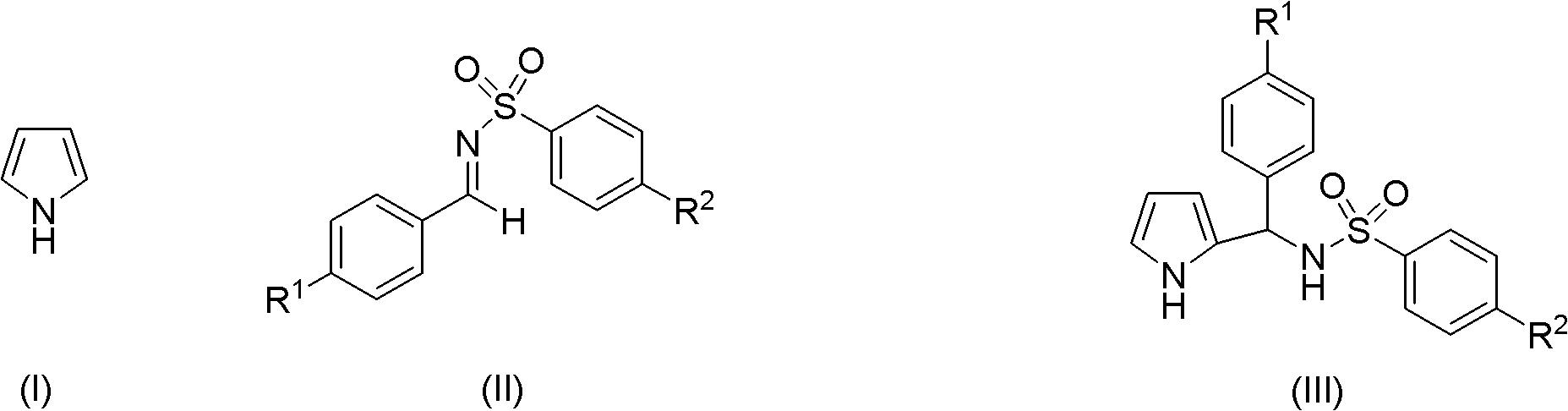
Preparation method of 2-pyrrole benzenylsulfonylamide compound
A technology for pyrrole sulfonamides and compounds, which is applied in the field of preparation of 2-pyrrole sulfonamide compounds, can solve the problems of poor selectivity, low operation yield, and easy production of by-products, etc., achieving good yield and safety for operators , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of benzenesulfonylimide compound:
[0026] Taking N-benzylidene-4-methylbenzenesulfonamide as an example, the preparation method is: 1mmol benzaldehyde (reactant A) and 1mmol 4-methylbenzenesulfonamide (reactant B), and 0.5mmol chloride Mix aluminum, grind and react at room temperature for 1.5 hours, after the reaction, add 20ml of saturated aqueous sodium chloride solution, extract with ethyl acetate, take the organic layer and dry it with anhydrous sodium sulfate and filter, take the filtrate and distill off the solvent, N-benzylidene-4-methylbenzenesulfonamide was obtained by recrystallization from ethanol.
[0027] The benzenesulfonylimide compounds used in the examples were prepared according to the above method steps, the difference was that the raw materials were changed, wherein reactant A and reactant B were shown in Table 1 below:
[0028] Table 1
[0029] Benzenesulfonylimides
Embodiment 1
[0031] N-(Phenyl(2-pyrrole)methyl)p-toluenesulfonamide
[0032]
[0033] Pyrrole (13.4mg, 0.2mmol), N-benzylidene-4-methylbenzenesulfonamide (103.6mg, 0.4mmol), indium trichloride (0.02mmol), methanesulfonic acid (0.04mmol) in tetrahydrofuran (0.6mL), reacted in an ice-water bath, detected the end point of the reaction with TLC, and reacted for 8 hours. After the reaction was completed, 5mL of water was added to the reaction solution, extracted with 20mL of dichloromethane, the organic layer was dried and filtered, and the filtrate was distilled to remove the solvent. Afterwards, column chromatography (petroleum ether: ethyl acetate = 3: 1) was refined, and TLC tracked and collected the eluent with an Rf value of 0.25 to 0.28, and the collected eluent was decompressed to remove the eluent to obtain the target product 40.5 mg, yield 62%, yellow solid.
[0034] 1 MR (500MHz, CDCl 3 ): δ8.59(s, 1H), 7.54-7.57(m, 2H), 7.18-7.23(m, 3H), 7.13-7.15(d, J=7.5Hz, 2H), 7.09-7.12(m,...
Embodiment 2
[0036] Refer to Example 1 for the operation, without adding indium trichloride, the others are the same as Example 1, and the yield is 8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com