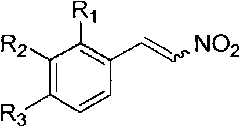
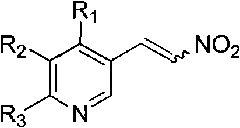
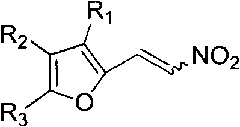
Method for synthesizing 4-aryl-4,5-dihydrofuran
A technology for dihydrofuran and derivatives, which is applied in the field of high-yield synthesis of 4-aryl-4, can solve the problems of low yield, poor substrate universality, complex synthesis process, etc., and achieve simple reaction process and low cost Low, easy-to-handle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0040] Embodiment 1-3 mainly investigates the influence of temperature of reaction, reaction time
[0041]
Embodiment 1
[0043] With β-nitrostyrene (0.2mol) 29.8g, acetylacetone (0.4mol) 40.0g, K 2 CO 3 (0.12mol) 16.6g, DMSO (200ml), was added to a 500ml reaction flask. After stirring and reacting at 30°C for 8 hours, the conversion of the nitrostyrene raw material was complete. Dilute hydrochloric acid (1 mol / L) was added to pH = 6, extracted with ethyl acetate, washed with water and saturated brine in turn once. Dry over anhydrous sodium sulfate, evaporate ethyl acetate, and separate by flash column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain the target product with a yield of 58%.
Embodiment 2
[0045] With β-nitrostyrene (0.2mol) 29.8g, acetyl lactone (0.4mol, 2eq) 40.0g, K 2 CO 3 (0.12mol, 0.6eq) 16.6g, DMSO (200ml), was added to a 500ml reaction flask, stirred and reacted at 80°C for 1 hour, and the conversion of the nitrostyrene raw material was complete. Dilute hydrochloric acid (1 mol / L) was added to pH = 6, extracted with ethyl acetate, washed with water and saturated brine in turn once. Dry over anhydrous sodium sulfate, evaporate ethyl acetate, separate by flash column chromatography (petroleum ether: ethyl acetate = 8: 1), yield 94%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com