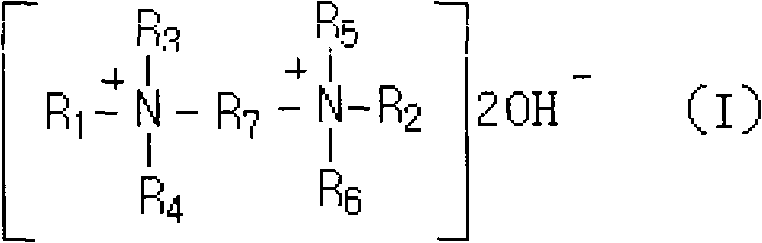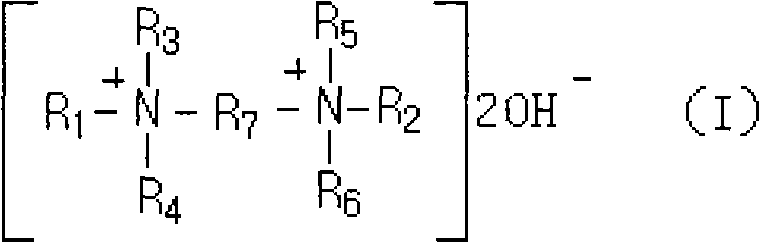Preparation method of 4-aminodiphenylamine
A technology for aminodiphenylamine and aniline, applied in the field of preparing 4-aminodiphenylamine, can solve the problems of slow hydrogenation rate, low recovery rate of tetramethylammonium hydroxide, low yield of 4-aminodiphenylamine, etc.
Active Publication Date: 2010-09-15
JIANGSU YANGNONG CHEM GROUP +1
View PDF14 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
(1) the selectivity of condensation reaction nitrobenzene and the yield of 4-aminodiphenylamine are not high;
(2) The hydrogenation rate of the alkali-containing condensation liquid is relatively slow;
(3) The stratification speed of the hydrogenation liquid is slow and there is emulsification;
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
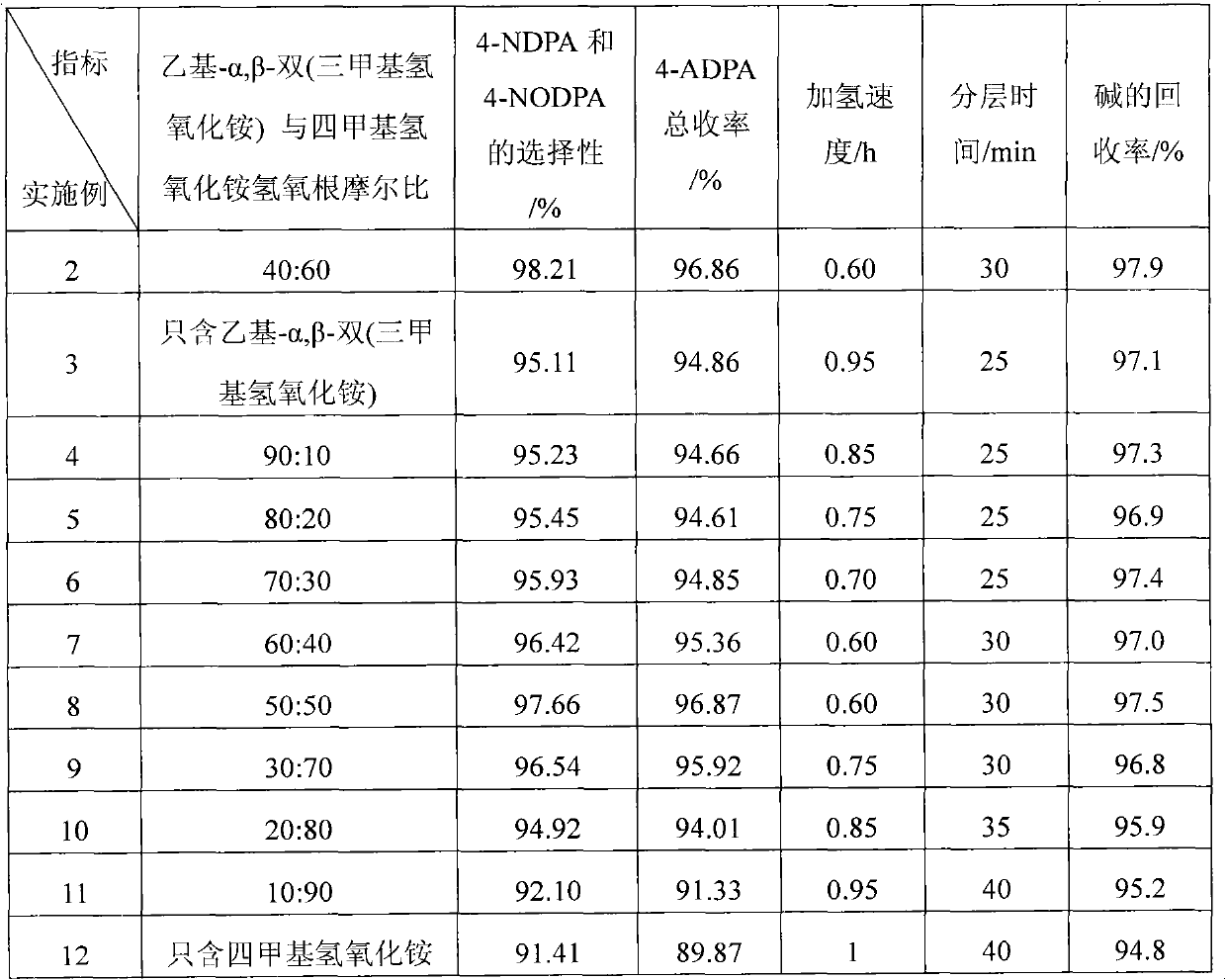
Embodiment 1
Embodiment 2
Embodiment 3~12
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a preparation method of 4-aminodiphenylamine, wherein a self-made mixture of bis-quaternary ammonium base and tetramethylammonium hydroxide with low price and good heat stability is used as a base catalyst for a condensation reaction of nitrobenzene and aniline. The bis-quaternary ammonium base has the structure of a general formula (I), wherein R1 and R2 are mutually independent alkyls of C1-C18 and preferentially of methyl, ethyl, n-propyl, isopropyl, allyl, butyl or dodecyl; R3, R4, R5 and R6 are mutually independent straight chain or branch chain alkyls of C1-C6 and preferentially of methyl, ethyl or propyl; and R7 is an alkylene or alkoxy substituted alkylene of C2-C6 and preferentially of ethylidene, propylidene, 2-methoxypropylidene, 2-ethyoxylpropylidene orbutylidene. The preparation method has the advantages that the selectivity of the nitrobenzene in the condensation reaction and the yield of the 4-aminodiphenylamine are high; the hydrogenation speedof a base-containing condensation liquid is high; a hydrogenation liquid has high layering speed and no emulsification phenomenon; and the base has good stability and is not easy to disintegrate, thereby the recovery rate of the base catalyst is improved and the production cost is reduced.
Description
technical field The present invention relates to a method for preparing 4-aminodiphenylamine, especially a method for preparing 4-aminodiphenylamine by mixing a novel self-made diquaternary ammonium base with tetramethylammonium hydroxide as a catalyst for the condensation reaction of nitroaniline method. Background technique 4-aminodiphenylamine (4-aminodiphenylamine), also known as RT base, is mainly used in the production of p-phenylenediamine rubber antioxidants 4010NA, 4020, etc. There are more than ten kinds of production methods for 4-aminodiphenylamine, and the currently industrialized methods mainly include aniline method, formanilide method, diphenylamine method and nitrobenzene method. These several methods all first synthesize 4-nitrodiphenylamine or 4-nitrosodiphenylamine, and then reduce to obtain 4-aminodiphenylamine. The nitrobenzene method produces 4-aminodiphenylamine, that is, nitrobenzene and aniline are condensed in the presence of a base catalyst to...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C211/54C07C209/36C07C209/00
Inventor 程晓曦许金来丁克鸿顾克军顾志强王秋萍
Owner JIANGSU YANGNONG CHEM GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com