Human immunoglobulin-like receptor mutant and application thereof
A technology for human immunoglobulins and mutants, applied in animal/human proteins, receptors/cell surface antigens/cell surface determinants, specific peptides, etc., can solve the problem of low ILT3 efficiency, inability to adapt to preservation, ILT3 extracellular domain Problems such as protein instability, to achieve the effect of improving stability and preparation efficiency, significant economic value and social significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1, Preparation of Human Immunoglobulin-like Receptor Mutants
[0037] 1. Bioinformatics analysis
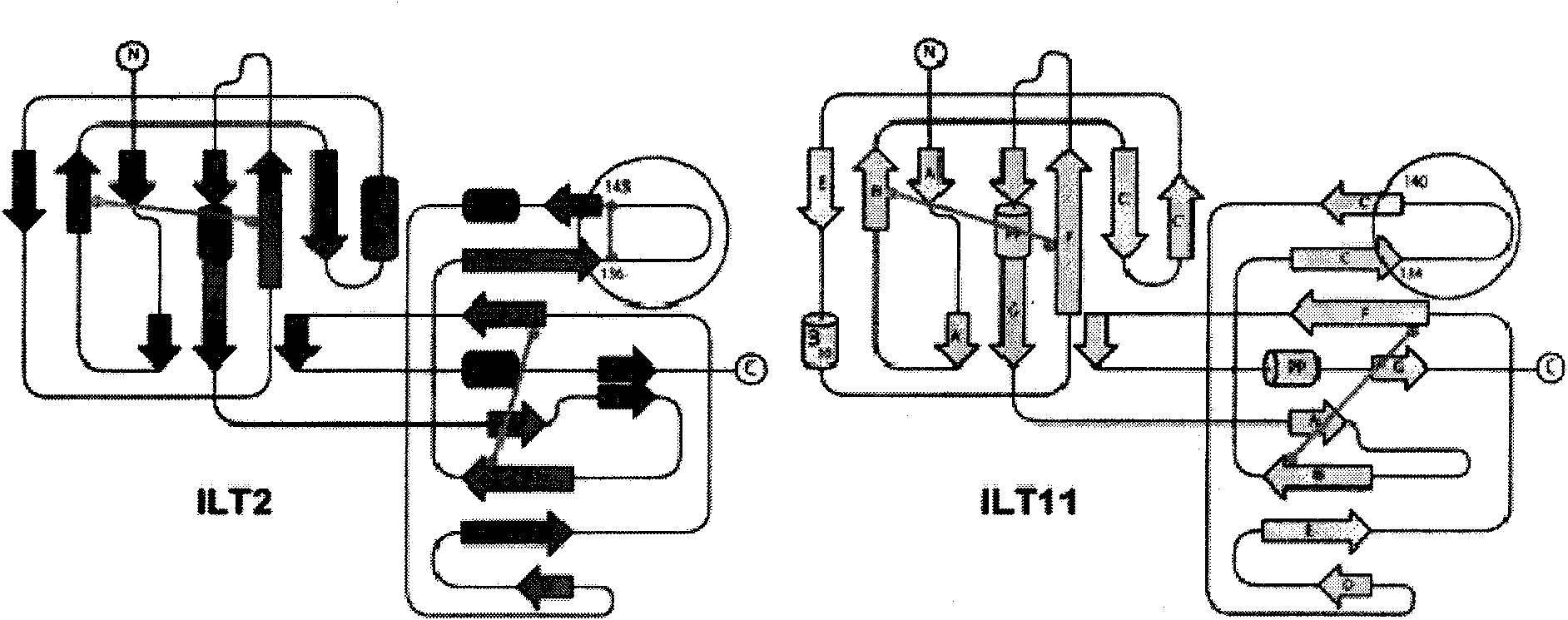
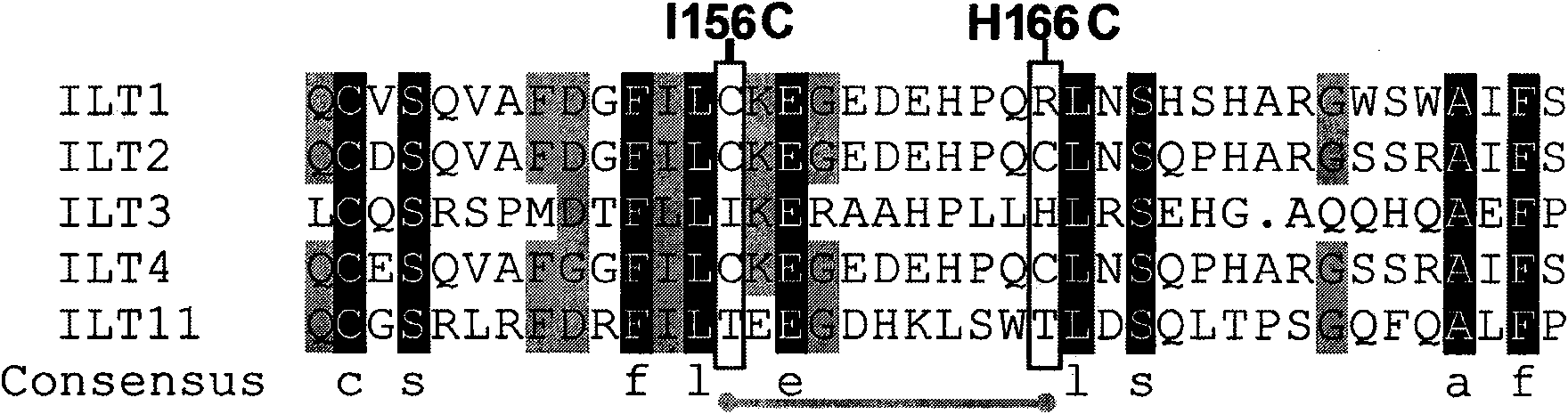
[0038] The analysis found that the flexible loop region of the Ig domain 2 of the ILT3 protein had an adverse effect on the structural rigidity of the entire protein. Sequence comparison analysis of ILT 2 / 4 / 11, a member of the ILT family, found that they have a common feature, that is, each immunoglobulin domain is composed of two disulfide bonds (disulfide bond 1 and disulfide bond 2) Fixed to form a stable folding mode, the two disulfide bonds are formed by two pairs of highly conserved cysteines. For members of ILT 2 / 3 / 4 / 11, except for two pairs of highly conserved cysteines, other cysteines are non-conserved. As long as the non-conserved cysteine exists, it always appears in pairs to form a disulfide bond (disulfide bond 3), and the position of the disulfide bond is fixed. ILT3 and ILT11 do not have this non-conserved disulfide bond, which makes the loop r...
Embodiment 2
[0074] Example 2, Determination of Physiological and Biochemical Properties of ILT3cc Protein
[0075] The purified ILT3cc protein and ILT3wt protein (control) prepared in Example 1 were identified as follows:
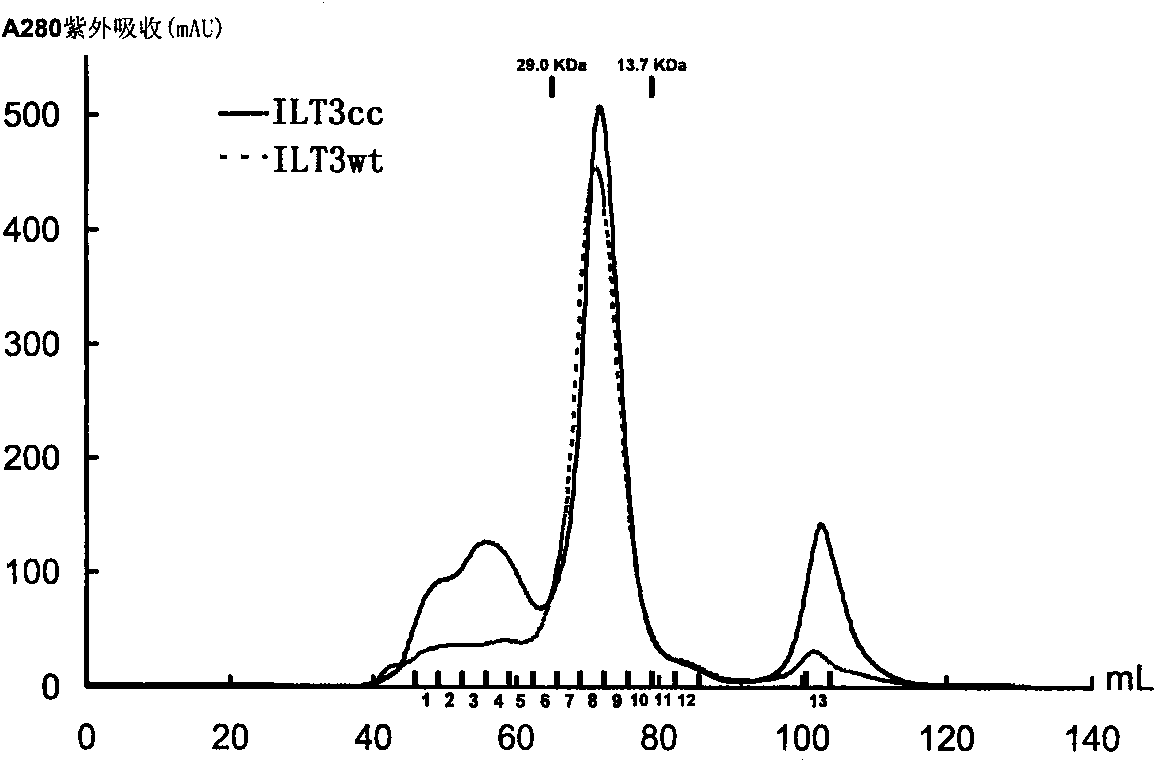
[0076] 1. Molecular sieve chromatography
[0077] ILT3cc protein and ILT3wt protein were purified with Superdex 75HR molecular sieve chromatography column (GE Healthcare) and concentrated. Molecular sieve chromatography see image 3 , the results showed that the properties of ILT3cc and ILT3wt solutions remained consistent.
[0078] After molecular sieve chromatography, the collected ILT3cc protein was subjected to SDS-PAGE, and the electropherogram is shown in Figure 4 . Place the ILT3cc protein and ILT3wt protein at 4°C for 24 hours, and then perform electrophoresis. The results are shown in Figure 5 . The results showed that after standing for 24 hours, compared with ILT3wt protein, the degradation of ILT3cc protein was significantly improved.
[0079] 2. P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com