Compound valsartan amlodipine besylate pharmaceutical composition and preparation method thereof
A technology of valsartan amlodipine besylate capsule and amlodipine besylate solid, applied in the valsartan amlodipine besylate pharmaceutical composition and its new preparation field, can solve the problem of amlodipine besylate amlodipine besylate Problems such as slow dissolution of clodipine, unstable product quality, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
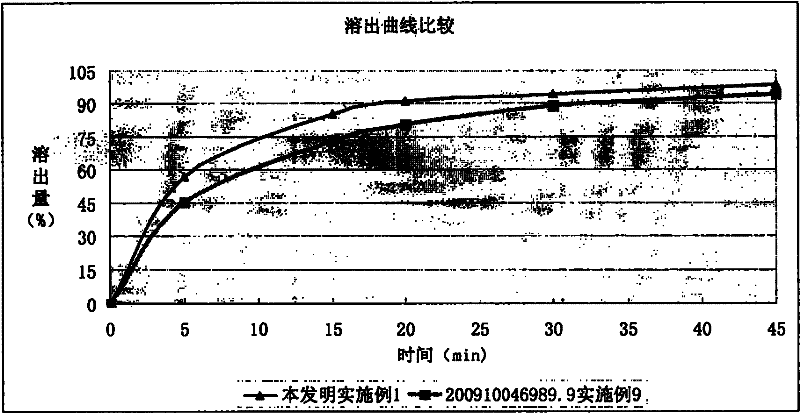
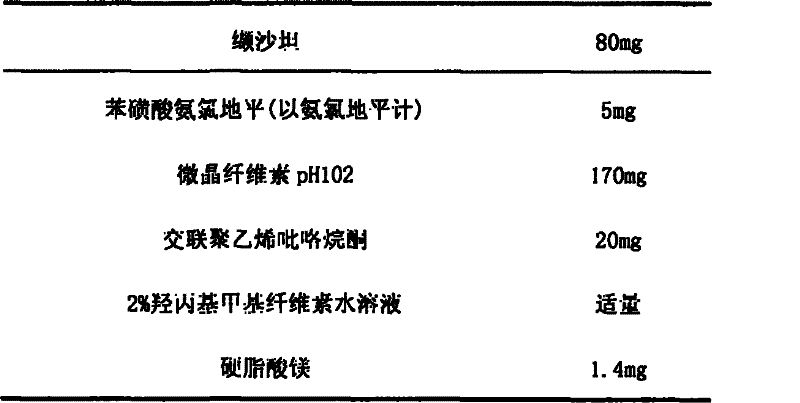
Image
Examples
Embodiment 1
[0075] The composition of the capsule prescription is as follows per 1000 capsules:
[0076] Valsartan 80g
[0077] Microcrystalline Cellulose 30g
[0078] Croscarmellose Sodium 10g
[0079] Appropriate amount of water
[0080] Amlodipine besylate solid dispersion 45.161g
[0081] Lactose 64.839g
[0082] Carboxymethyl Starch Sodium 10g
[0083] Appropriate amount of water
[0085] Composition of amlodipine besylate solid dispersion
[0086] Amlodipine besylate 6.944g
[0087] Povidone K30 34.72g
[0088] Polyoxyethylene castor oil 3.497g
[0089] 1) Preparation of amlodipine besylate solid dispersion: Weigh amlodipine besylate, povidone K30 and polyoxyethylene castor oil respectively, add absolute ethanol, stir to dissolve, evaporate under reduced pressure to remove absolute ethanol , evacuated, dried overnight, ground through a 100-mesh sieve, and set aside.
[0090] 2) Preparation of amlodipine besylate granules: Weigh the amlodipi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com