Application of 4-benzothiopheneaminoquinazoline derivative in preparing tumor treatment medicine
A benzothiophene aminoquinazoline, tumor drug technology, applied in the research field of anti-tumor drugs, can solve the problems of difficulty, no anti-tumor activity evaluation, impossible application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
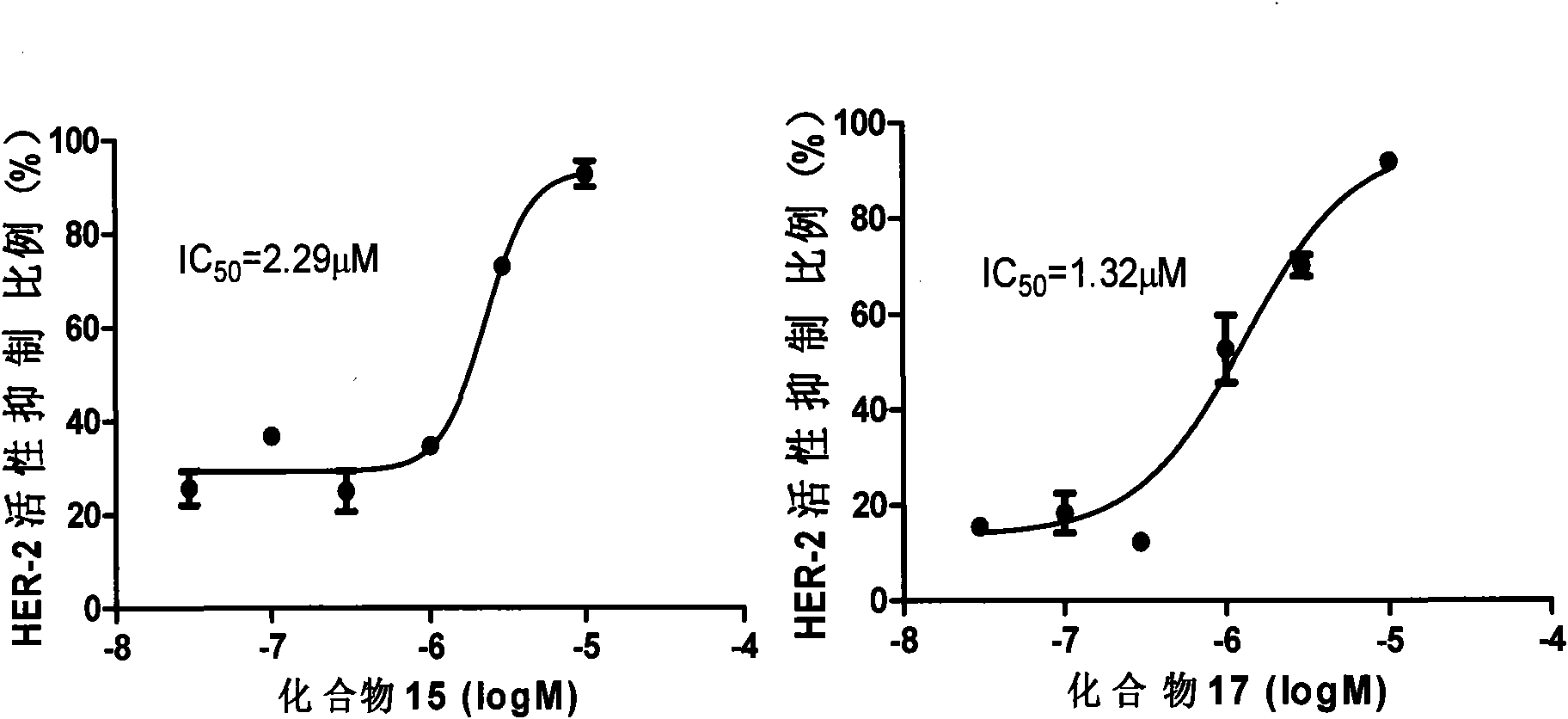
[0045] Example 1 Compound 1-20 inhibits receptor tyrosine kinase
[0046] EGFR, HER-2, c-MET and other kinase detection kits from Cell Signaling Technology were used to measure the effects of various concentrations of compounds 1-20 and two positive control compounds Gefitinib and Erlotinib on the above receptor tyrosine according to the product instructions. Inhibitory activity of amino acid kinase (inhibition rate and half inhibitory concentration IC50, figure 1 ). Table 2 lists the inhibitory rates of 10.0 μM compounds 1-20 and two positive control compounds on three kinases, and the data are the average values of two independent experiments. As can be seen from the experimental results, the inhibitory activity of compounds 11-20 (except compound 16) to HER-2 is equivalent to or better than the positive control compound, and the inhibitory activity to c-MET is better than the positive control compound; Compound 12 , 15, 17, 18 and 19 had slightly less inhibitory activit...
Embodiment 2
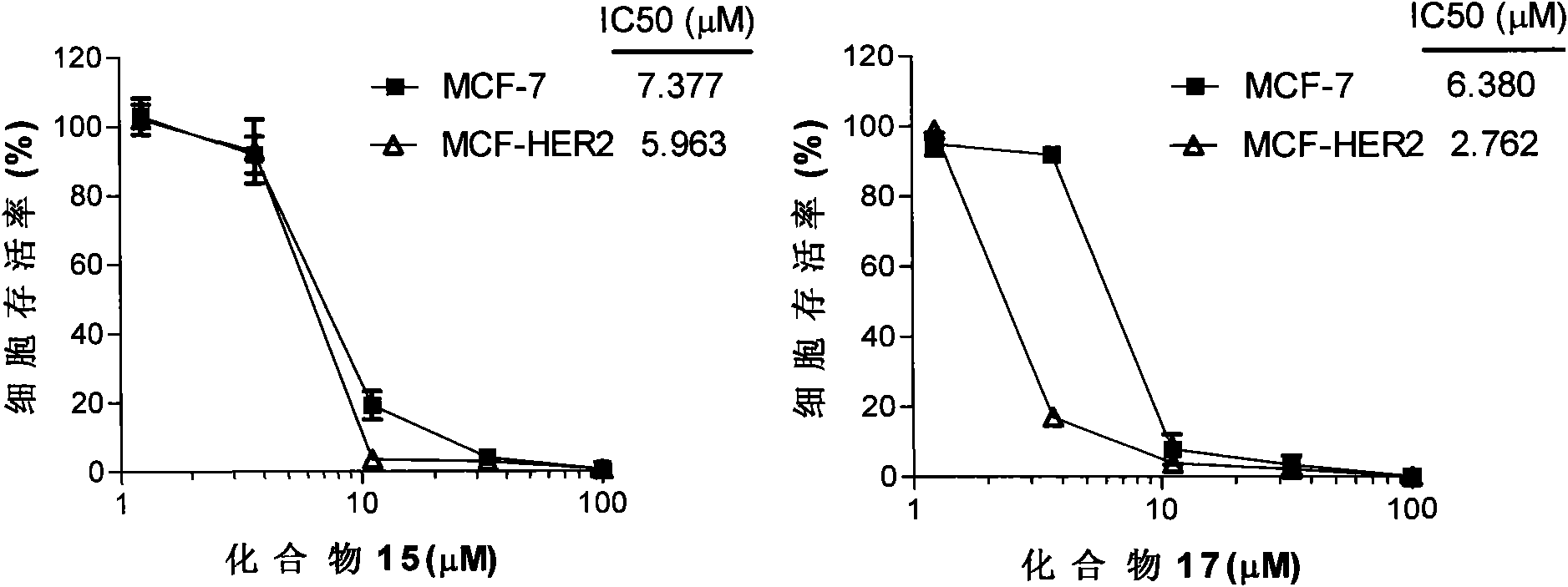
[0047] Example 2 Compound 1-20 inhibits tumor cell growth
[0048] Six different human tumor cell lines were treated with compounds 1-20 and two positive control compounds Gefitinib and Erlotinib. Two human pancreatic cancer cells, Miapaca2 and Panc1, two human prostate cancer cells, DU145 and PC3, and two human lung cancer cells, A549 and NCI-H661, were seeded in 96-well cell culture plates, and compound 1-20 and Two positive control compounds. At 37°C, 5% CO 2 After culturing in an incubator for 72-96 hours, the survival rate of the cells was detected with WST-8 reagent. The half maximal inhibitory concentrations (IC50) of 22 compounds on 6 kinds of human cell lines are shown in Table 2, and the data are the average value of three independent experiments. It can be seen from the experimental results that most of the compounds exhibit tumor cell growth inhibitory activity superior to the positive control compounds.
[0049] HER-2-negative breast cancer cells MCF-7 and HER...
Embodiment 3
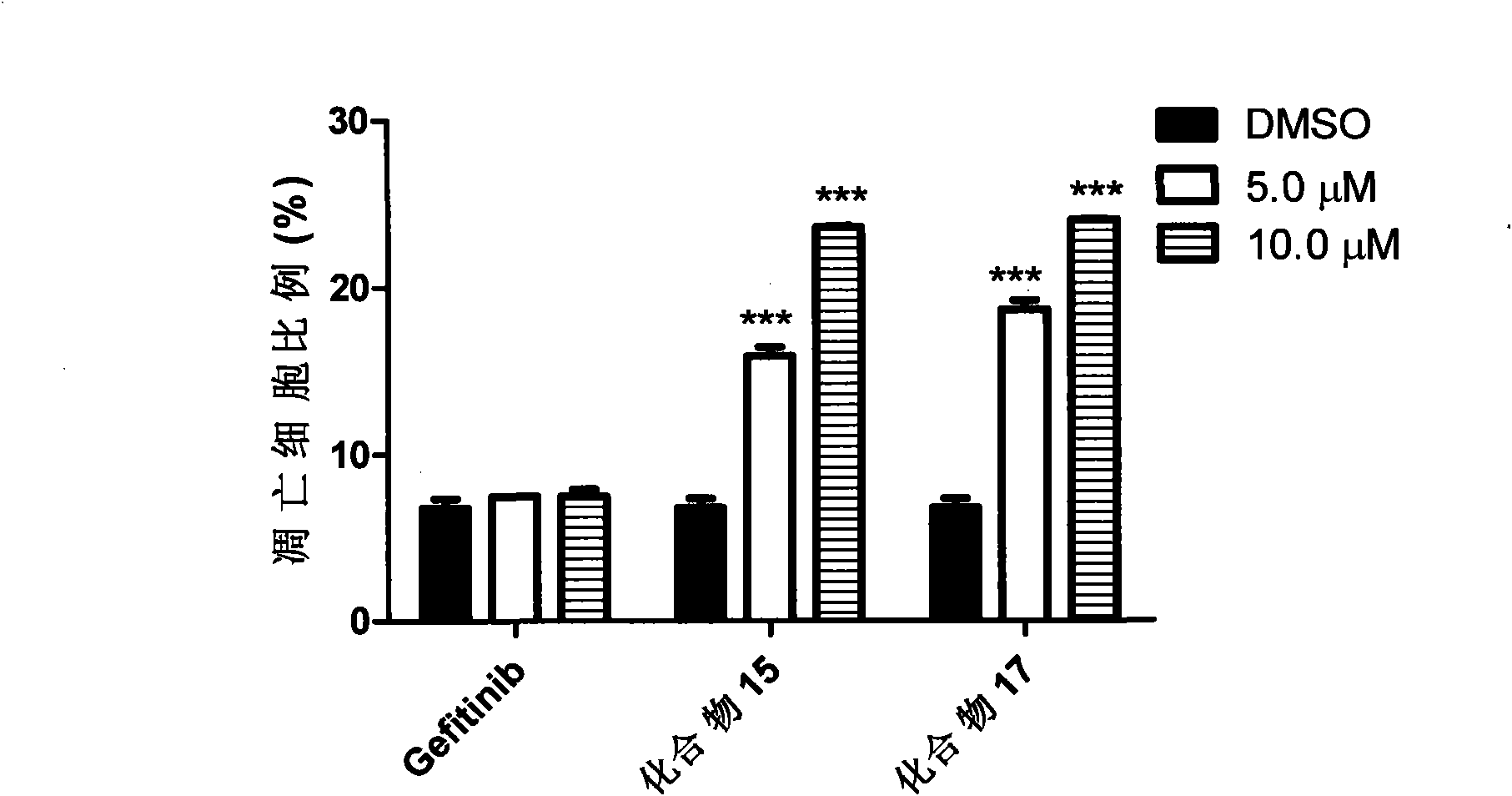
[0050] Example 3 Compounds 15 and 17 Induce Pancreatic Cancer Cell Miapaca2 Apoptosis
[0051] Pancreatic cancer cells Miapaca2 were inoculated in 6-well cell culture plates, and the cells were treated with different concentrations of compounds 15, 17 and Gefitinib for 48 hours, then the cells were collected, stained with propidium iodide (PI), and analyzed by flow cytometry. dead cells. The experimental results showed that the percentage of apoptosis in Miapaca2 cells increased with the concentration of compounds 15 and 17, and 10.0 μM of compounds 15 and 17 could induce about 25% of the apoptosis, while Gefitinib at the same concentration could hardly induce apoptosis ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com