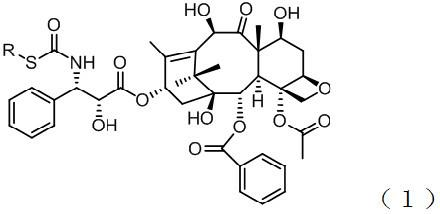
New application of thio-taxane derivatives
A technology of taxanes and derivatives, applied in drug combinations, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
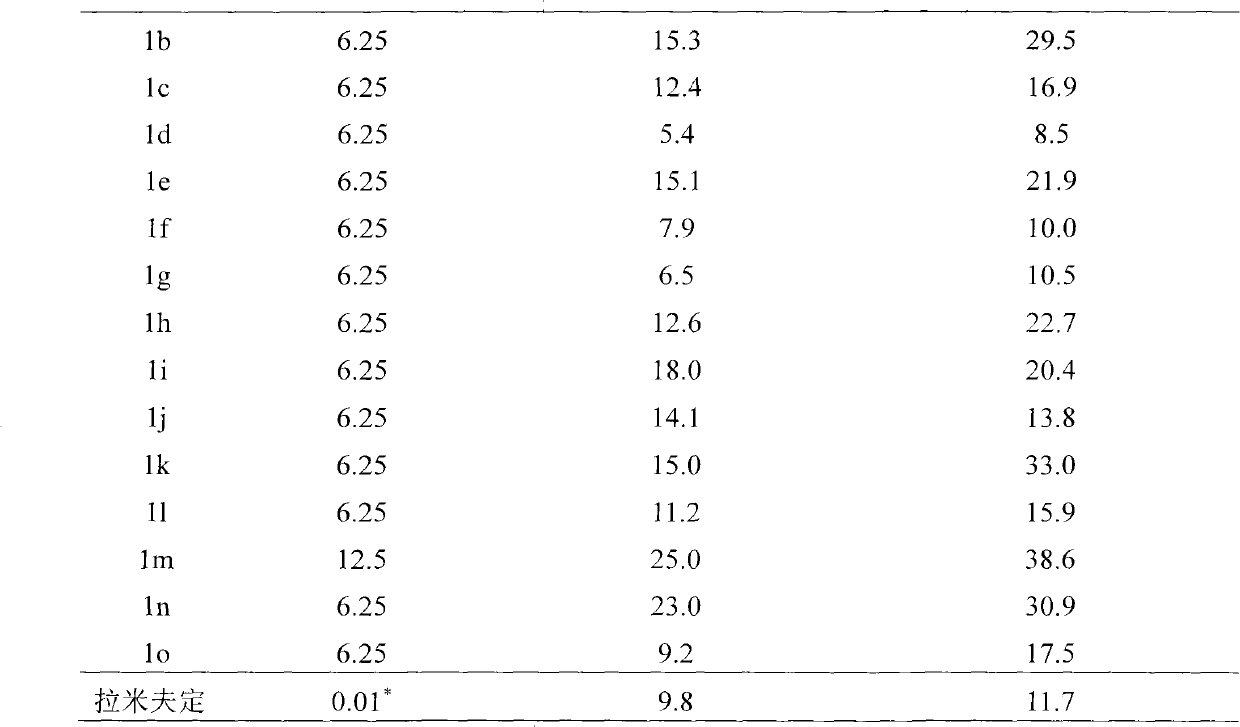
Image
Examples
Embodiment 1
[0027]
[0028] Take a 100mL three-neck flask, burn it to dryness, blow nitrogen, and add BTC (3.82g) at 0°C, then add dry CH 2 Cl 2 (40mL), after the BTC is fully dissolved, add ethyl mercaptan (2.41mL), slowly drop Et 3 N (4.48mL), a white precipitate was formed. After the dropwise addition was completed, the liquid was filtered at room temperature, and the insoluble matter was filtered off from the reaction liquid. The obtained thioacyl chloride solution was directly used in the next reaction without any treatment. Get a 100mL three-necked flask, add paclitaxel intermediate compound 2 (500mg), and use CH 2 Cl 2 (10mL) was dissolved, and the excess acid chloride was slowly added dropwise in an ice-water bath. After the dropwise addition was completed, the liquid was stirred at room temperature to stop the reaction, extracted with ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was removed. Analysis (el...
Embodiment 2
[0037]
[0038] Compound 1g (R is isopropyl), 1 H NMR (300MHz, CDCl 3 ): δ1.11(s, 3H, 17-CH 3 ), 1.14 (d, 3H, J=7.2Hz, 1″-CH 3 ), 1.19 (d, 3H, J=7.2Hz, 3″-CH 3 ), 1.24(s, 3H, 16-CH 3 ), 1.72 (s, 3H, 19-CH 3 ), 1.83(s, 3H, 18-CH 3 ), 2.25 (d, 2H, J=7.5Hz, 14-CH 2 ), 2.35 (s, 3H, OAc), 1.83 and 2.53 (2m, 2H, 6-CH 2 ), 3.49 (m, 1H, 2″-CH), 3.86 (d, 1H, J=6.6Hz, 3-CH), 4.17 and 4.30 (2d, 2H, J=8.7Hz, 20-CH 2 ), 4.21 (m, 1H, 7-CH), 4.45 (s, 1H, 2′-OH), 4.72 (br s, 1H, 2′-CH), 4.93 (d, 1H, J=9.0Hz, 5 -CH), 5.30 (s, 1H, 10-CH), 5.59 (d, 1H, J = 9.0Hz, 3'-CH), 5.65 (d, 1H, J = 6.6Hz, 2-CH), 6.24 ( t, 1H, J=8.7Hz, 13-CH), 6.52(d, 1H, J=8.7Hz, -CONH-), 7.33(m, 1H, p-Ph), 7.39(m, 4H, o-Ph and m-Ph), 7.50(t, 2H, J=7.5Hz, m-OBz), 7.62(t, 1H, J=7.5Hz, p-OBz), 8.11(d, 2H, J=7.5Hz, o -OBz); ESIMS m / z 832.1 [M+Na] + .
[0039] Compound 1h (R is tert-butyl), 1 H NMR (300MHz, CDCl 3 ): δ1.11(s, 3H, 17-CH 3 ), 1.24(s, 3H, 16-CH 3 ), 1.35(s, 9H, t-Bu), 1.71(s, 3H, 19-CH 3 ), 1...
Embodiment 3
[0045] Compound 1m (R is benzyl), 1 H NMR (400MHz, CDCOCD 3 ): δ1.14(s, 3H, 17-CH 3 ), 1.19 (s, 3H, 16-CH 3 ), 1.71 (s, 3H, 19-CH 3 ), 1.86(s, 3H, 18-CH 3 ), 2.20 (m, 2H, 14-CH 2 ), 2.36(s, 3H, OAc), 1.82and 2.44(2m, 2H, 6-CH 2 ), 3.77 (s, 1H, 2′-OH), 3.90 (d, 1H, J=7.0Hz, 3-CH), 4.07 (d, 2H, J=4.0Hz, 1″-CH 2 ), 4.15 (d, 2H, J=5.1Hz, 20-CH 2 ), 4.29 (m, 1H, 7-CH), 4.35 (s, 1H, 10-OH), 4.68 (d, 1H, J=4.8Hz, 2′-CH), 4.94 (d, 1H, J=9.5 Hz, 5-CH), 5.21 (s, 1H, 10-CH), 5.55 (d, 1H, J=6.0Hz, 3'-CH), 5.66 (d, 1H, J=7.0Hz, 2-CH) , 6.18(t, 1H, J=8.8Hz, 13-CH), 7.25(m, 1H, p-Ph), 7.28(t, 1H, J=7.7Hz, p-1″-Ph), 7.39(m , 4H, o-Ph and m-Ph), 7.40 (t, 2H, J=7.2Hz, m-1″-Ph), 7.47 (d, 2H, J=7.3Hz, o-1″-Ph), 7.56(t, 2H, J=7.5Hz, m-OBz), 7.64(t, 1H, J=7.3Hz, p-OBz), 7.90(m, 1H, J=5.2Hz, -CONH-), 8.10( d, 2H, J=7.3Hz, o-OBz); ESIMS m / z 880.3 [M+Na] + .
[0046] Compound 1n (R is p-methylphenyl), ESIMS m / z 880.3[M+Na] + .
[0047] Compound 1o (R is p-methoxyphenyl), ESIMS m / z 896.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com